Summary
Background
Withdrawal of treatment is a common therapeutic problem in patients with long‐standing remission of inflammatory bowel disease.
Aims
To evaluate the relapse rate in patients with quiescent inflammatory bowel disease after cessation of biologic or immunomodulator therapy.
Methods
We searched five databases for studies evaluating disease relapse after withdrawal of monotherapy or a drug from combination therapy in Crohn's disease or ulcerative colitis. In meta‐analysis, risk ratios (RR) were calculated with 95% confidence intervals (CI).
Results
Ten randomised controlled trials (587 patients) were included in the meta‐analysis, and another nine studies in systematic review. Withdrawal of immunomodulator monotherapy resulted in a significantly higher risk of relapse within 24 months of follow‐up compared to ongoing therapy in Crohn's disease, but not in ulcerative colitis (RR = 2.06, CI: 1.53‐2.77 and RR = 1.39, CI: 0.85‐2.26, respectively). Trial sequential analysis indicated that further studies with similar design are unlikely to change the significant association on relapse rates after withdrawing immunomodulator monotherapy in patients with Crohn's disease. Discontinuation of an immunomodulator from combination with biologics did not show a higher risk of relapse than continuation of both drugs (RR = 1.30, CI: 0.81‐2.08). The relapse rate increased after withdrawal of biologic monotherapy, whereas contradictory results were observed after biologic withdrawal from combination regimens.
Conclusion
Continuing immunomodulator monotherapy should remain the preferred approach among patients with Crohn's disease, although long‐term toxicity is a concern. Further randomised controlled trials are warranted in ulcerative colitis and on combination regimens including biologics.
1. INTRODUCTION
Inflammatory bowel disease (IBD)—comprising Crohn's disease (CD) and ulcerative colitis (UC), as the two main types—is chronic condition of the gastrointestinal tract with a relapsing and remitting pattern. CD is characterised by transmural inflammation and the chance of stricture development at any segment of the gastrointestinal tract. 1 CD is more likely to be associated with disease‐related complications (eg abscesses, strictures) and extraintestinal manifestations than UC. 2 Although UC is a superficial mucosal inflammation of the colon, it can also cause several complications, such as fulminant colitis and increased risk of colorectal cancer. 3 The risk of surgery 1, 5 and 10 years after diagnosis of CD was 16.3%, 33.3% and 46.6%, while that in UC is 4.9%, 11.6% and 15.6% respectively. 4
The therapeutic regimen of CD and UC bears several similarities. Medical treatments include 5‐aminosalicylates for UC, and corticosteroids immunomodulators (IM, eg azathioprine, methotrexate or mercaptopurine) for both UC and CD. Biologic therapies have been available for more than 20 years to provide patients with moderate‐to‐severe disease with the best therapeutic option for the induction and maintenance of remission. 5 , 6 In clinical practice, three major classes of biologics are approved for IBD: tumour necrosis factor (TNF) alpha antagonists, integrin and interleukin‐12/23 antagonists. 7 In addition to the assessment of the severity and activity of the disease, and to risk stratification, the optimal treatment decision involves individual and financial considerations. 8 , 9 The lifetime treatment strategy focuses not only on the induction and maintenance of remission but also on complete mucosal healing to prevent complications of the disease. Treatment with IMs and biologics improves the quality of life, reduces corticosteroid requirements and its consequences, but toxicity may occur. 10 When treated with IMs or biologics, moreover, with the combination of both agents, serious concern exists about opportunistic infections (eg tuberculosis, histoplasmosis). Studies of the CESAME cohort have highlighted the risks and consequences of IMs concerning the increased risk of lymphoproliferative, skin and urinary tract malignancies. 11 , 12 , 13 , 14
Despite the consensus and guidelines for remission maintenance IBD therapies, our knowledge of withdrawing effective therapies in remission is uncertain. 15 Several rationales for stopping treatment exist, such as reducing total health care costs, adverse events (AE) or serious adverse events (SAE), and patient‐specific factors are also considered (eg adherence to treatment, life events [pregnancy, breastfeeding], long‐lasting remission). 7
Recently, The European Crohn's and Colitis Organisation published a consensus on stopping treatment, called 'exit strategy'. 15 In UC patients with mild clinical course and complete mucosal healing, dose reduction in 5‐aminosalicylates can be considered but 5‐aminosalicylates should be continued in the long term to reduce the risk of relapse and colorectal cancer. 15 In the case of IM monotherapy in CD, an early cohort study found a similar relapse rate after 4 years in remission, regardless of whether IM therapy was continued or not. 16 In CD, recent randomised controlled trials (RCTs) and observational studies with different follow‐up periods showed an increased relapse rate after IM withdrawal. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 Unfortunately, fewer studies were performed in UC than in CD. 25 , 26 In three RCTs, IM withdrawal in CD patients treated in combination with biologic therapy resulted in a similar relapse rate compared to that of continued combination therapy. 27 , 28 , 29 In a recent meta‐analysis, the overall risk of relapse after anti‐TNF withdrawal was 30%‐40% at 1 year, and 50% at 2 years, but there is a lack of controlled, high‐quality studies in this area. 30
The aims of the present study were to systematically review and meta‐analyse the efficacy and safety of discontinuation of IMs or biologics in both UC and CD.
2. METHODS
We reported our meta‐analysis following the rules of the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) Statement (Table S1). 31 The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO, registration ID: CRD42020155848).
2.1. Search strategy
Our search was conducted from inception until 5 September 2020 in the following five electronic databases: MEDLINE (via PubMed) (http://www.ncbi.nlm.nih.gov/pubmed), Embase (https://www.embase.com), the Central Cochrane Register of Controlled Trials (CENTRAL) (http://www.cochranelibrary.com), Web of Science (www.webofknowledge.com) and Scopus (https://www.scopus.com/). In Scopus and Web of Science, title and abstract fields were used; on the other sites, all fields were used, and no restrictions were applied. Manual search was also performed in the reference lists of the included studies to identify additional studies.
We set up a search query based on the PICO formula. We examined the population (P) of patients with IBD in remission after de‐escalation or withdrawal of effective therapy. Only studies dealing with patients in stable remission on therapy were included. Withdrawal was defined as the complete discontinuation of the drug. De‐escalation of treatment was defined as either decreasing the dose or increasing the therapeutic interval of the drug. Analysing the intervention (I) item, four groups were defined: I1 withdrawal of IM monotherapy, I2 withdrawal of an IM from the combination therapy, I3 withdrawal of biologic monotherapy and I4 withdrawal of a biologics from the combination therapy. The comparators (C) were patients with IBD on ongoing medication. In our meta‐analysis, we searched for biologic agents included infliximab (IFX), adalimumab, certolizumab, golimumab, vedolizumab and ustekinumab; and IMs (azathioprine, mercaptopurine and methotrexate). The primary outcomes (O) consisted of the relapse rate after 1 and 2 years of follow‐up. The secondary outcomes were AEs and SAEs, and we also aimed to identify the predictive factors of relapse. The full‐length search key can be found in the appendix (Table S2).
2.2. Study selection and eligibility
After the systematic search and import of all references into a reference management software (EndNote X8, Clarivate Analytics), duplicates and overlapping records were removed. The potentially eligible records were screened based on title and abstract, independently by two authors (DD and PP). The same two authors screened the full texts or abstracts of the remaining articles for eligibility. A third author (PS) resolved discrepancies when necessary.
RCTs and cohort studies comparing the relapse rate after stopping an effective treatment versus ongoing therapy according to the pre‐defined PICO were eligible for inclusion. Findings of cohort studies were narratively synthesised in systematic review only. Conference abstract was included as well. We excluded clinical trials recruiting patients with active disease without reaching remission.
2.3. Data extraction
Two independent investigators (DD and PP) extracted the data separately, and disagreements were resolved by consensus. The following predefined data were extracted from each study: first author, year of publication, study design, the form of publication (full‐text/abstract) and the number of participating centres. Sample size and gender distribution, age at discontinuation of the drug, predictive factors of relapse (smoking, disease phenotype, C‐reactive protein, haemoglobin, steroid‐free treatment period), IBD type and the definition of remission and relapse, and received drugs (before and after discontinuation of IM or biologic therapy) were also recorded. Most importantly, data on disease activity were collected to assess remission or relapse of the disease. For safety analysis, AEs and SAEs were also collected and categorised following the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use‐Good Clinical Practice (ICH‐GCP) consensus guidelines. 32
2.4. Risk of bias assessment
The revised Cochrane risk‐of‐bias tool for randomised trials (RoB2) was used for the risk of bias assessment of the included RCTs. 33 Bias was assessed in five different domains: randomisation process, deviations from intended interventions, missing outcome data, measurement of the outcome and selection of the reported results. After evaluation, the risk of low, some concerns and high bias were indicated with green, yellow and red signs respectively.
The Risk of Bias In Non‐Randomized Studies–of Interventions (ROBINS‐I) tool was used to assess the risk of bias of the included observational studies. 34 Seven different items of bias were assessed: confounding, selection of participants, classifications of interventions, deviations from intended interventions, missing data, measurement of outcomes and selection of the reported outcome. At the end, an overall bias assessment was performed. After evaluation, low, moderate, serious, critical risk of bias or no information was indicated with light green, light blue, yellow, dark green and dark blue respectively.
The two authors (DD and PP) first assessed risk of bias within the selected studies independently, and disagreements were resolved by the opinion from a third investigator (PS). Results of the risk of bias assessment were discussed when assessing the limitations of the individual studies.
2.5. Quality of evidence
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) was used for estimating the quality of evidence for the primary outcome of the meta‐analysis. 35 Outcomes were tested based on five criteria: risk of bias, inconsistency, indirectness, imprecision and publication bias. The overall quality of the evidence for each outcome was graded as high, moderate, low or very low. Grading was performed independently by two authors (DD, SzK), and disagreements were resolved by a third author (PS).
2.6. Statistical analysis
Data analysis was based on the intention‐to‐treat principle. Risk ratios (RRs) were calculated for dichotomous outcomes with 95% confidence intervals (CI). The random‐effects model was used for all analyses with DerSimonian‐Laird estimation. 36 Statistical heterogeneity was assessed using Cochrane's Q, the I2 statistics and chi2. According to the Cochrane Handbook for Systematic Reviews of Interventions, heterogeneity was interpreted moderate between 30% and 60%, substantial between 50% and 90% and as considerable above 75%. 37
We planned to investigate the predictive factors of relapse by pooling RRs or hazard ratios; however, data were seldom and not truly comparable.
We planned to evaluate publication bias by Egger's test and visual inspection of the funnel plot.
We also performed Trial Sequential Analysis (TSA) for the primary outcomes to determine whether further randomised trials with similar design are needed. 38 Using this methodology, the information size of trials can be combined with the threshold of statistical significance. Reliable evidence is obtained with crossed trial sequential monitoring boundaries (red lines in the corresponding figures). Statistical analyses were performed with Stata 16 (StataCorp LLC, College Station, Texas, USA) and Trial Sequential Analysis Program version 0.9 beta (available from www.ctu.dk/tsa).
Subgroup analyses were performed to analyse if the application of placebo (placebo and placebo‐free studies after therapy withdrawal in the intervention arm) and disease type (CD and UC) affect the relapse rate.
A leave‐one‐out sensitivity analysis was performed to test if the removal of any study changes the association.
3. RESULTS
3.1. Study selection
After the selection of the 46,673 records, 10 RCTs were eligible for inclusion in meta‐analysis. In systematic review, an additional RCT with insufficient data for meta‐analysis 39 and 8 cohort studies were discussed. The study selection is detailed in Figure 1.
FIGURE 1.
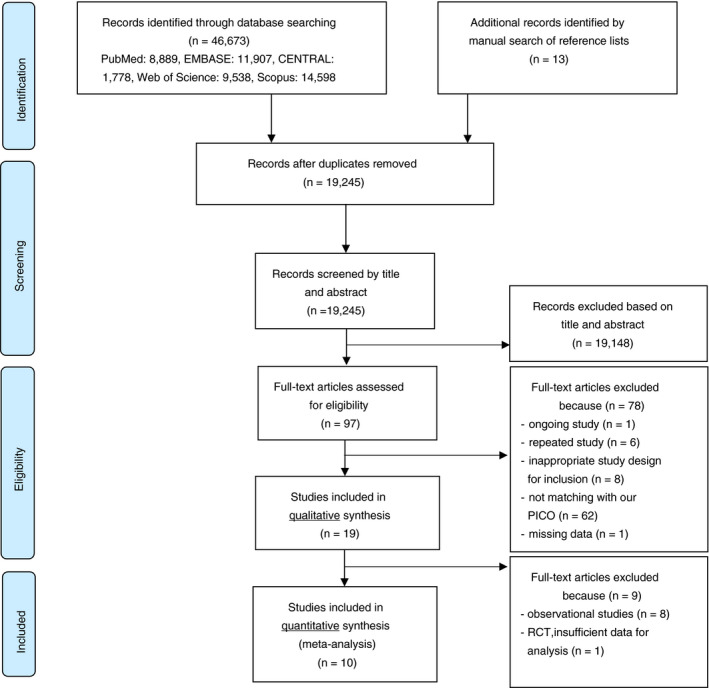
Flow chart of study selection
3.2. Characteristics of the studies included
The characteristics of the studies included are summarised in Table 1. The studies were published between 1978 and 2020. Eleven of 19 studies were RCTs. Studies reported data from Europe (n = 12), America (n = 2), Africa (n = 1) and Asia (n = 4). Patients with CD were studied in 13 studies 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 29 , 40 , 41 , 42 and patients with UC in 4 studies, 25 , 26 , 39 , 43 and 2 studies recruited both CD and UC population. 44 , 45 Clinical relapse was determined in 14 17 , 18 , 19 , 20 , 21 , 22 , 23 , 40 , 41 , 42 , 43 , 44 and remission in 15 studies, 17 , 18 , 19 , 20 , 21 , 22 , 23 , 29 , 39 , 40 , 41 , 43 , 44 while endoscopic activity was reported in only 3 studies. 25 , 43 , 44 Studies used different types of indices to define clinical remission and relapse, such as the Crohn's Disease Activity Index, 46 the Harvey‐Bradshaw Index 47 and the Mayo score. 48 In the UC studies, the Mayo endoscopic subscore and a grading scale based on the publication of Baron et al were used to define endoscopic remission. 48 , 49
TABLE 1.
Characteristics of the studies included
| Author, year | Study type (number of centres) | IBD type | Number of patients in the IG | Male n (%) in the IG |
Age at intervention (years) in the IG |
Number of patients in the CG | Male n (%) in the CG | Age at intervention (years) in the CG | Drug | Follow‐up (months) | Definition of remission | Definition of relapse |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies for withdrawal of IM monotherapy | ||||||||||||
| Candy et al, 1995 23 | RCT (1) | CD | 19 | 11 (36.70) | 57.5 (48‐64) b | 33 | 7 (21.20) | 33.9 (15‐60) b | AZA | 12 | Clinical (CDAI < 150) | Clinical (CDAI > 175) |
| O’Donoghue et al, 1978 18 | RCT (1) | CD | 27 | 11 (40.70) | 40.5 (22‐65) c | 24 | 11 (45.80) | 40 (21‐78) c | AZA | 12 | Clinical (constant clinical state) | Clinical (deterioration requiring change in treatment) |
| Feagan et al, 2000 22 | RCT (7) | CD | 36 | 22 (61.10) | 34 ± 2 d | 40 | 16 (40) | 32 ± 2 d | MTX | 10 | Clinical (absence of the need for prednisone and CDAI < 150) | Clinical (∆CDAI = 100 point/ prednisone/anti‐metabolite use) |
| Hawthorne et al, 1992 25 | RCT (5) | UC | 34 | 22 (64.70) | 44 (19‐82) c | 33 | 12 (36.40) | 44 (23‐73) c | AZA | 12 | Clinical and endoscopic (absence of symptoms without steroids and sigmoidoscopy: grade 0 or 1) | Clinical (worsening symptoms) |
| Kim et al, 1999 21 |
Prospective cohort (1) |
CD | 36 | 22 (61.10) | 31.1 (14.80‐68.50) c | 84 | 33 (39.30) | 37.4 (15.30‐81.20) c | MP | 6‐150 | Clinical (HBI < 4) | Clinical (HBI ≥ 4) |
| Lémann et al, 2005 17 | RCT (12) | CD | 43 | 18 (41.90) | 36 ± 11 d | 40 | 19 (47.50) | 40 ± 14 d | AZA | 18 | Clinical (CDAI < 150) | Clinical (CDAI ≥ 250, or CDAI between 150 and 250, ∆CDAI = 75 points/need for surgery) |
| Lobel et al, 2004 26 |
Prospective cohort (1) |
UC | 22 | 10 (45.40) | 42 (25‐29) b | 39 | 20 (51.30) | 51 (20‐73) b | MP | 40 (4‐344) b | Clinical (at least 4 of 5: absence of diarrhoea/abnormal endoscopic findings/gross blood in the stool, patient's subjective assessment, doctor assessment) | Clinical (recurrence of the original symptoms) |
| Sokol a et al, 2009 24 | Retrospective cohort (NA) | CD | 47 | NA | NA | 94 | NA | NA | AZA | 60 | NA | NA |
| Vilien et al, 2004 20 | RCT (NA) | CD | 15 | NA | 47 (23‐73) b | 14 | NA | 33 (22‐63) b | AZA | 12 | Clinical | Clinical (∆CDAI ≥ 75 and CDAI > 150) |
| Wenzl et al, 2014 19 | RCT (2) | CD | 26 | 13 (50) | 39.30 ± 11.80 d | 26 | 16 (61.50) | 38.20 ± 11.90 d | AZA | 24 | Clinical (without the need of oral prednisone, CDAI < 150) | Clinical (increased disease activity with the need of oral corticosteroid) |
| Studies for withdrawal of an immunomodulator from combination therapy | ||||||||||||
| Choi a et al, 2010 42 | Retrospective cohort (1) | CD | 7 | NA | NA | 15 | NA | NA |
AZA+ IFX |
12 | NA | Clinical (requiring surgery or corticosteroid treatment) |
| Hisamatsu et al, 2019 29 | RCT (NA) | CD | 29 | 22 (75.86) | 35 ± 14 d | 23 | 18 (78.26) | 35 ± 11 d | AZA/ MP + ADA | 12 | Clinical (Corticosteroid‐free remission, CDAI < 150) | NA |
| Roblin et al, 2017 44 | RCT (1) | CD, UC | 26 | 12 (46.10) | 31 (19‐63) f | 28 | 16 (57.10) | 30 (20‐60) f | AZA + IFX | 13 | Clinical and endoscopic (CD: CDAI < 150, FC < 250 ug/g; UC: Mayo score < 3, endoscopic Mayo subscore 0‐1 and stool blood subscore 0) | Clinical |
| Van Assche et al, 2008 27 | RCT (NA) | CD | 40 | 19 (47.50) | 35.40 ± 10.80 d | 40 | 17 (42.50) | 35.60 ± 9.50 d | IM + IFX | 24 | NA | NA |
|
Yerushalmy‐Feler, et al, 2018 45 |
Retrospective cohort (NA) | CD, UC | 32 | NA | NA | 32 | NA | NA | IM + anti‐TNF | 19.1 (6.5‐24) c | NA | NA |
| Studies for withdrawal of biologic monotherapy | ||||||||||||
| Fiorino a et al, 2016 43 | Retrospective cohort (12) | UC | 111 | NA | 35.6 (29.10‐47.50) f | 82 | NA | 35.8 (26.20‐46.10) f | IFX | 12 | Clinical and endoscopic (Mayo subscore ≤ 2; Mayo endoscopic subscore ≤ 1) | Clinical and endoscopic (Mayo score ≥ 2 with rectal bleeding and endoscopic subscore ≥ 1) |
| Kobayashi a et al, 2020 39 | RCT (23) | UC | 46 | NA | NA | 46 | NA | NA | IFX | 11 | Clinical | NA |
| Studies for withdrawal of a biologic from combination therapy | ||||||||||||
| Chauvin et al, 2014 41 | Retrospective cohort (1) | CD | 54 | 24 (44.40) | 33 (23‐24) f | 38 | 11 (28.9) | 30 (24‐42) f | IFX + AZA or MTX | 47.1 (4.4‐110.2) f | Clinical, HBI < 4 | Clinical, HBI > 4 |
| Wynands et al, 2008 40 | Retrospective cohort (1) | CD | 16 | 9 (56.20) | 10.3 ± 2.50 d , g | 20 | 12 (60) | 10.70 ± 2.20 d , g | IFX + AZA or MTX | 12 | Clinical, HBI < 5 | Clinical, HBI > 5 |
Abbreviations: IG, intervention group; CG, control group; RCT, randomized controlled trial; CD, Crohn’s disease; UC, ulcerative colitis; CDAI, Crohn’s disease activity index; HBI, Harvey‐Bradshaw index; FC, fecal calprotectin; AZA, azathioprine; MTX, methotrexate; MP, mercaptopurine; IFX, infliximab; ADA, adalimumab; NA, not available.
Abstract.
Median (range).
Mean (range).
Mean ± SD.
Median (IQR).
At diagnosis.
Studies were classified into four groups based on the discontinued drug: withdrawal of IM monotherapy (n = 10), 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 IM from combination with biological therapy (n = 5), 27 , 29 , 42 , 44 , 45 biological monotherapy (n = 2) 39 , 43 and a biologics from combination with IM (n = 2) groups. 40 , 41 Altogether, the most commonly used IM was azathioprine in 12 studies, 17 , 18 , 19 , 20 , 41 , 42 , 44 followed by mercaptopurine in 5 21 , 26 , 27 , 29 , 41 and methotrexate in 3 studies. 22 , 27 , 41 IFX was examined as a biologic in 6 withdrawal studies, 27 , 40 , 41 , 42 , 43 , 44 adalimumab was used only in 1 study after IM withdrawal from combination regimen. 29
Sixteen of 19 studies compared drug discontinuation to ongoing therapy, while 3 of 19 studies, where IM was withdrawn, compared placebo in the intervention group to ongoing medication in the control group. 17 , 18 , 22 A dose reduction or an increase in the therapeutic interval of the drug was found in only 1 study, 44 so that we were unable to create a 'de‐escalation' subgroup in meta‐analysis.
Difference in the length of stable remission on therapy was considerable across the studies, with a duration of remission of at least 3 to 42 months; the longest remission period was in the study of Lémann et al 17 Time to relapse ranged from 6 to 150 months in the retrospective cohort studies. In the study of Kim et al, the follow‐up extended up to 150 months, 21 while Feagan et al reported only 10 months of follow‐up. 22 Of the 16 studies, only 3 mentioned re‐treatment strategy and its results. 22 , 39 , 41
3.3. Result for withdrawal of immunomodulator monotherapy
Seven of 10 RCTs, including a total of 334 and 67 patients with CD and UC, respectively, assessed the rate of relapse after therapy withdrawal compared to continued therapy. 17 , 18 , 19 , 20 , 22 , 23 , 25 The follow‐up time ranged from 10 to 24 months across the studies. There was a significantly higher relapse rate after stopping IM compared to ongoing therapy (RR = 1.85, 95% CI: 1.44‐2.38, P < 0.001), with negligible between‐study heterogeneity (I 2 = 0%, P = 0.832) (Figure 2). Subgroup analyses for CD and UC revealed a significantly higher relapse rate in CD but not in the single study of UC (RR = 2.06, 95% CI: 1.53‐2.77, P < 0.001, and RR = 1.39, 95% CI: 0.85‐2.26, P = 0.189 respectively) (Figure 2A). In a subgroup analysis, the relapse rates were significantly higher after discontinuation of the IM therapy in studies with or without placebo control (RR = 1.95, 95% CI: 1.29‐2.97, P = 0.002; I 2 = 0%, P = 0.832 and RR = 1.79, 95% CI: 1.31‐2.46, P < 0.001; I 2 = 0%, P = 0.582 respectively) (Figure 2B).
FIGURE 2.
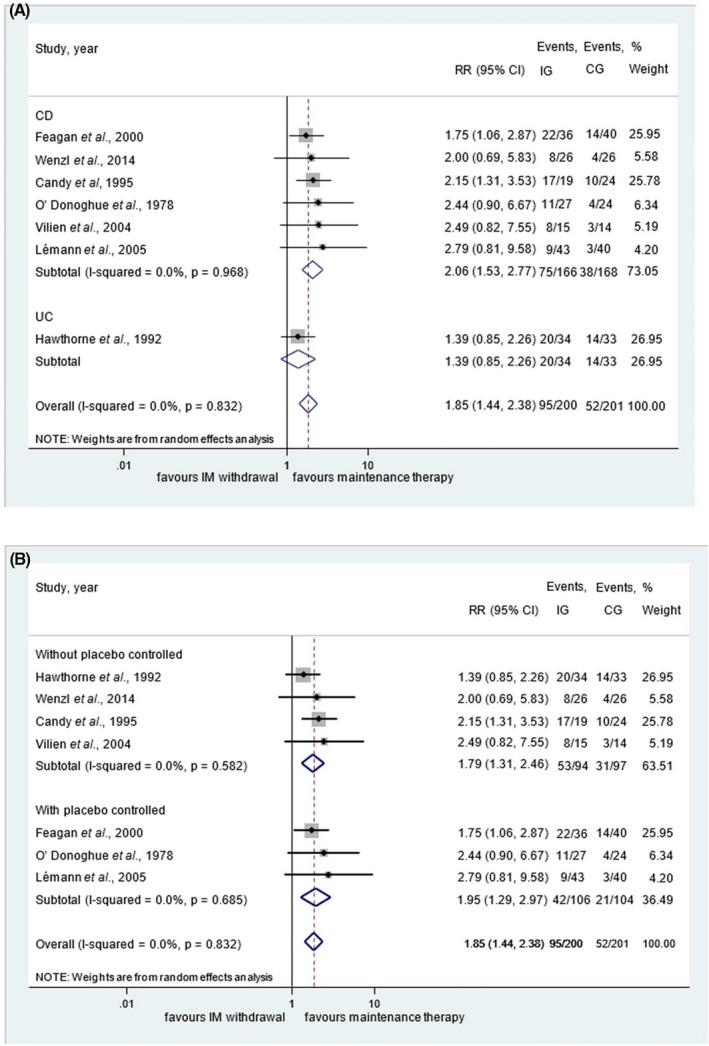
Results for withdrawal of immunomodulator monotherapy within 24 months of follow‐up compared to ongoing treatment. Subgroup analysis of patients with Crohn's disease and ulcerative colitis (A) and subgroup analysis of studies with placebo and without placebo control after drug discontinuation (B)
However, when we analysed only the five RCTs with a uniform follow‐up of 12‐months, the relapse rate remained significantly higher after stopping IM monotherapy compared to control patients treated with continued therapy (RR = 1.81, 95%CI: 1.38—2.36, P < 0.001; I 2 = 0.0%, P = 0.682) (Figure 3).
FIGURE 3.

Results for withdrawal of immunomodulator monotherapy within 12 months of follow‐up compared to ongoing treatment
No subgroup analysis could be performed with methotrexate or mercaptopurine due to the low number of studies available. To test the robustness of the associations, we performed a post hoc leave‐one‐out sensitivity analysis by iteratively removing one study at a time and recalculating the summary RR. The summary RRs remained stable (Figure S1).
In the three observational studies analysed, increased disease activity and relapse rates were found after withdrawal of IMs. 21 , 24 , 26
3.4. Results for withdrawal of an immunomodulator from combination therapy
Only 3 of 5 RCTs, including a total of 186 patients with IBD in stable remission on IM in combination with IFX or adalimumab, analysed the relapse rate after the withdrawal of an IM from combination therapy. 27 , 29 , 44 No statistically significant difference was observed between the groups (RR = 1.30, 95% CI: 0.81—2.08, P = 0,269; I2 = 0.0%, P = 0.641) (Figure 4). Sensitivity analysis showed that the removal of any study does not change the direction of the main association (shown in Figure S1).
FIGURE 4.
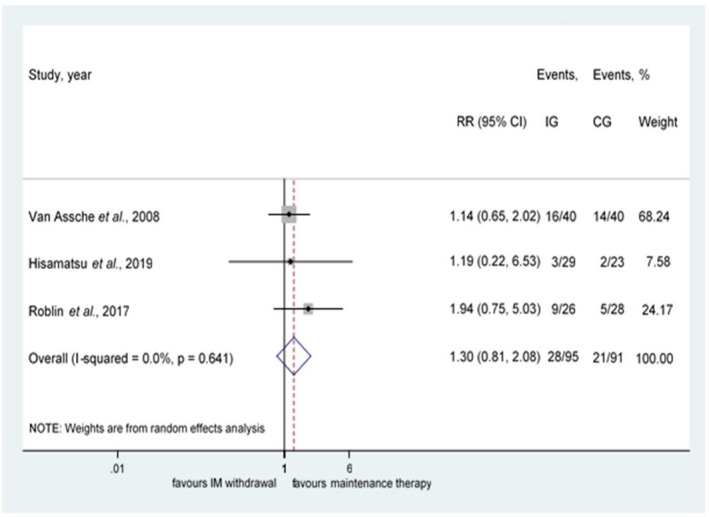
Results for withdrawal of an immunomodulator from combination therapy
In two retrospective cohort studies, no significant differences were found between the groups after IM withdrawal from combination regimen. 42 , 45
3.5. Results for withdrawal of biologics from mono‐ or combo‐therapy
Although our primary aim was to include withdrawal of biologic monotherapy and a biologic from IM combination treatment in meta‐analysis, we were unable to create this group due to insufficient data.
One retrospective cohort study, published in abstract form, compared 111 UC patients who discontinued IFX monotherapy to 82 patients with scheduled IFX therapy. Patients who stopped IFX showed a higher risk of relapse after therapy withdrawal (hazard ratio = 3.41, 95% CI: 1.88‐6.20, P < 0.001). Rates of hospitalisation and colectomy were not different between the groups. 43 One RCT published in abstract form examined the relapse rate after withdrawal of IFX monotherapy. Relapse rates at week 48 were 19.6 and 45.7% in the groups in which IFX was continued and discontinued respectively. 39
Two retrospective studies analysed the withdrawal of a biologic from the IM combination regimen. 40 , 41 In the study by Wynands et al, children with CD in long‐standing clinical remission discontinuing IFX treatment experienced relapse within 1 year in 75% of patients (12/16), of whom 58% (7/12) underwent surgery or 75% (9/12) started steroid therapy and required IFX re‐treatment (7 of 9 steroid users). 40 However, in the study of Chauvin et al, no significant difference was observed after IFX withdrawal in CD (hazard ratio = 0.73, 95% CI: 0.41‐1.30, P = 0.29). 41
3.6. Safety analysis
Of the 19 studies analysed, 10 reported the rate of AEs or SAEs. 17 , 18 , 19 , 44 In most of the articles, the exact number of events in the different groups was not reported. Therefore, no meta‐analysis could be performed. In the studies, common AEs were iron deficiency, infections (such as Clostridium difficile infection), abdominal symptoms, arthralgia, rash, insomnia and infusion reactions, whereas SAEs included lymphoma, leukopenia, pancytopenia, myelodysplasia and death (Table S3).
3.7. Predictive factors of relapse
We intended to collect the potential risk factors from the included studies to predict relapse. Multivariate analyses of possible predictive factors were performed only in 4 studies. 17 , 19 , 26 , 41 Based on the differences between the examined factors and cut‐off values, pooled results could not be calculated in our meta‐analysis. The predictive factors are detailed in Table S4.
3.8. Trial sequential analysis
During TSA of the IM monotherapy withdrawal group within a 1‐year follow‐up, the cumulative Z‐curve (blue line in Figure S2A) crossed the vertical boundary (red line in Figure S2A), indicating that the required information size was achieved in the case of patients with CD (n = 114). There is no need to include further studies with similar design because results are unlikely to change (Figure S2A).
The TSA was carried out on the IM monotherapy group within a 2‐year follow‐up as well. The results showed a statistically significant difference between the groups and reached the required information size according to the cumulative z‐curve (blue line in Figure S2B) (n = 156). According to the TSA, further studies with similar design are unlikely to change the significant results on relapse rates after withdrawing IM monotherapy in CD (Figure S2B).
TSA proved to be inconclusive in the analysis in the UC subgroup on IM monotherapy withdrawal (Figure S2C), in that on IM withdrawal from combination treatment (Figure S2D) and in that on biologic monotherapy withdrawal (Figure S2E) due to insufficient data.
3.9. Risk of bias assessment
Assessments of the risk of bias that included RCTs are shown in Figure S3A. In the RCTs, the randomisation process was sufficiently described in only 6 of 11 studies. Deviations from the intended interventions were recorded in 7 of 9 studies. The study of Kobayashi et al, published as a conference abstract, was judged to carry a high risk due to the limited information available. 39 All studies were judged to have a low risk for missing outcome data, except for the study of Van Assche et al, in which many patients discontinued the trial due to AEs and SAEs, 27 and for the study of Kobayashi et al due to missing data. 39 The 'measurement of the outcome' domain was rated the best, with 8 of 9 studies being judged low risk. After evaluating the overall bias, 4 studies were assessed with a low risk of bias, 17 , 19 , 22 , 44 and 6 were rated as having some concerns risk of bias 18 , 20 , 23 , 25 , 27 , 29 and 1 intervention was evaluated with high risk of bias. 39
Observational studies were included in the systematic review part of the article, and the results of the risk of bias assessment are presented in Figure S3B. Conference abstracts carried the highest risk of bias. 24 , 42 , 43 In the full‐text articles, the pre‐intervention domains, including confounding and selection bias, were mostly assessed with serious risk of bias. Only the prospective studies from Kim and Lobel were classified as having a low and moderate risk of bias. 21 , 26 The intervention and the post‐intervention bias was considered to carry low risk in prospective studies and moderate risk in retrospective studies. Regarding the overall risk of bias, retrospective studies were rated as carrying serious risk of bias, 40 , 41 , 43 and prospective studies as carrying low 21 or moderate risk of bias. 26
3.10. Quality of evidence
Based on the GRADE analysis, the quality of evidence for relapse rates was rated at very low to low. The GRADE assessment of the main outcome (relapse rate) showed low quality of evidence for the analyses of patients with CD and UC in the IM monotherapy withdrawal group within 24 months of follow‐up. In the 12‐month follow‐up studies, relapse rate showed low quality of evidence. Very low quality of evidence was rated for the main outcome in the IM or biologic withdrawal from combination regimen groups, and low quality of evidence was rated for the main outcome in the biologic monotherapy withdrawal group. The GRADE evidence profile is shown in Figure S4.
3.11. Publication bias
Due to the low number of included studies, we could not investigate publication bias by means of the Egger's test or the visual inspection of the funnel plots. 50
4. DISCUSSION
Since IBD is a chronic, relapsing and progressive inflammatory state of the gastrointestinal tract, potent immunosuppressive drugs and biological agents are used alone or in combination for treatment. However, in addition to the effectiveness of different drugs, toxicity, healthcare costs and national regulations should also be considered. 51 The feasibility of therapy withdrawal after medically induced remission is a common scientific question, and several systematic reviews have recently addressed this issue. 7 , 15 , 30 , 51 , 52 , 53
Firstly, we assessed the effect of withdrawal of IM monotherapy, where the results showed an almost twice as high chance of relapse at both 1 and 2 years after therapy withdrawal than with continued therapy. A twofold relapse rate was detected only in the CD subgroup but not in UC. This result should be interpreted with caution as only one RCT analysed patients with UC. 25 Regardless of the 3 types of IM (methotrexate, mercaptopurine and azathioprine), all individual studies reported a higher chance of relapse after drug cessation. In the studies analysed, the duration of stable treatment appeared to be heterogeneous; hence, our results question the validity of the traditional 'three‐to‐four‐year cessation rule'. Although there is a well‐known high placebo effect in IBD, no difference was detected between the two subgroups in which placebo or no placebo was administered after IM discontinuation.
Secondly, when IM was withdrawn from a combination with anti‐TNF treatment, the relapse rate was not significantly higher. In the study of van Assche et al, no clinical benefit was found in continuing combo therapy. However, dual therapy maintained low C‐reactive protein levels and high IFX trough levels. 27 Furthermore, concomitant IM therapy, regardless of the type of IM, influenced the pharmacokinetics of antibodies against IFX and adalimumab as well. 54 , 55 A recent meta‐analysis confirmed that patients receiving combination therapy were less likely to develop antibodies. 56 Higher levels of antibodies to IFX (≥8.0 μg/mL) predicted subsequent loss of treatment response and an increased risk of infusion reactions. 57 However, the trough level of anti‐TNF agents during combination therapy was independent of concomitant IM therapy. 58 In the study of Roblin et al, a dose de‐escalation group was also created, where azathioprine dose reduction resulted in better outcomes than direct therapy withdrawal, eg stable median trough level, more favourable pharmacokinetics, and appeared as effective as the continuation of azathioprine with the full dose. 44 In combination therapy, a reduced dose of azathioprine may reduce the production of neutralising anti‐TNF antibodies, thereby providing a lower chance of developing AE and SAE. Although IM withdrawal from combination regimen carries a higher risk of anti‐drug antibody formation, their effect on clinical outcomes may take longer than a year to become apparent.
The positive effect of adalimumab and IM combo‐therapy is still debated. In the study by Matsumuto et al, adalimumab monotherapy and adalimumab + azathioprine combo therapy worked equally effective, but the rate of AEs was lower in the monotherapy group. 59 Only one study included in our meta‐analysis (DIAMOND2) applied adalimumab as a withdrawn biologic agent; in this study, trough levels of adalimumab were not significantly different between the groups after azathioprine withdrawal from combination treatment. 29 The immunogenicity of newer biologics appears to be very low (4.2% against ustekinumab in the IM‐UNITI trial 60 and 3.7%‐4.1% against vedolizumab in the GEMINI trials). 61 , 62
Despite these apparent benefits of combo therapy with IFX, IM withdrawal from combination with biologics remains a preferred approach of long‐term treatment to avoid toxicity, but balancing between AEs of drugs and disease progression is unavoidable in patients with severe inflammation and complications. 15 Discontinuation of IM from combination regimens seems to be suitable in patients with long‐standing stable remission treated with other biologics than IFX.
Thirdly, in the retrospective study of Fiorino et al, in which IFX monotherapy was withdrawn, a higher relapse rate was observed in the intervention group. 43 Other uncontrolled cohort studies also reported an increased relapse rate after the withdrawal of a biological agent. 40 , 41 In a recent RCT, relapse rates were higher after withdrawal of IFX monotherapy than that during maintenance IFX. 39 In a meta‐analysis by Gisbert et al, relapse rates at 12 months were 40 and 28% for CD and UC respectively. 30 In a retrospective study by Steenholdt et al, the majority of patients who discontinued IFX while in remission relapsed over time (88% of patients with CD at 10‐year and 60% of patients with UC at 4.5‐year follow‐up). 63 In two small retrospective studies, conflicting results were reported on the biologic withdrawal from the combination regimen. 40 , 41 Thus, the withdrawal of biologic agents from both monotherapy and combination therapy remains an issue. Research to solve unresolved questions is already underway, eg the ongoing RCT of Chapparo et al evaluates the relapse rate after withdrawal of a previous biologic therapy (IFX or adalimumab) from combination regimens in patients with CD or UC who achieved clinical remission. 64 Future work will also focus on relapse rates following biologic therapy withdrawal in specific patient groups (such as perianal CD). 65 The three‐arm SPARE study compares IFX scheduled maintenance with or without IMs (azathioprine, methotrexate or mercaptopurine) and IM alone in terms of relapse rate after patients with luminal CD achieved remission for at least 8 months with combination therapy of IFX and IM. 66
Although a distinction between predictive factors for relapse would be useful, none of the included studies was able to determine the exact IBD subpopulation in which drug withdrawal could be safe and low risk. Factors associated with a higher relapse rate following IM monotherapy withdrawal were elevated C‐reactive protein or low haemoglobin levels, active smoking and high‐risk disease phenotype (eg perianal, extensive disease). 17 , 19 , 26 , 41
Patients with high‐serum anti‐TNF trough levels appear to be at an increased risk of relapse, ie these patients may probably need to maintain therapy to avoid relapse. 67 On the other hand; low, undetectable anti‐TNF trough levels may identify patients with a lower chance of relapse to discontinue treatment. 68 Using a series of predictive factors, the time to relapse can be calculated as described by Ben‐Horin et al. In this study, patients at low relapse risk were in remission for up to 24 months. 68 Restarting with the same drug after a drug holiday could be effective and safe, but there is a higher risk of developing immunogenicity, infusion reactions and loss of response. 68 In the case of re‐induction, lack of antibodies and concomitant IM therapy may shorten the time to therapeutic response. 69
Since the goal of IBD therapy is to achieve and maintain clinical and endoscopic remission, 5 , 6 endoscopic assessment should be considered when deciding to withdraw the drug. Withdrawal of biologics in a study population not selected based on endoscopic findings may result in lower remission rates. Mucosal healing seems to reduce the risk of relapse after discontinuation of anti‐TNF agents. 67 Assessment of mucosal healing and disease flare‐up can be performed not only by invasive endoscopic examination but also by using faecal calprotectin as a non‐invasive marker. 70 Unfortunately, the majority of studies included in our meta‐analysis only assessed relapse rates after clinical but not endoscopic remission. However, more recent data suggest that a lower relapse rate can be expected in the presence of endoscopic or histological remission after drug withdrawal. 30 , 71 After discontinuation of anti‐TNF in CD, a previous meta‐analysis showed a 42% relapse rate after clinical remission, in contrast to 26% when endoscopic remission was also achieved. 30 Similar results were observed in patients with UC, more patients experiencing clinical and endoscopic relapse than clinical relapse alone (58 and 42%, respectively) during the median 13‐month follow‐up period. 71 Moreover, a recent study confirmed the superiority of histological remission over histologically active disease in terms of relapse rate. 72
Our meta‐analysis has several limitations suggesting caution in interpreting the results. Firstly, we were unable to create more homogenous groups for meta‐analysis in terms of remission duration. Secondly, the number of studies analysing patients with UC was low, confirming the need for further controlled verification studies in this area. Thirdly, our pre‐defined secondary outcomes could not be met due to the lack of studies. Fourthly, in most studies, clinical criteria without ruling out active inflammation with objective tools (eg calprotectin, endoscopy or cross‐sectional imaging) were used to define remission. There were studies that did not report a precise definition of remission and relapse. Fifthly, the data on IM withdrawal from combination therapy were scarce so that we were unable to reach sufficient statistical power (see results of TSA). Finally, we could not evaluate other biologics than IFX as withdrawn drug (eg adalimumab, vedolizumab or ustekinumab) as no studies have been published in this field.
In summary, the present meta‐analysis confirms that the withdrawal of IM monotherapy increases the risk of relapse in patients with quiescent CD. According to the GRADE approach, the certainty of evidence is low, so that further studies may change the results. However, in TSA, the statistical power reached the required level, meaning that future studies with similar design will be unlikely to change the results. Results from UC and combination treatment groups should be treated with caution due to the low number of studies without high quality of evidence. The present findings may highlight the importance of this topic and the need for further RCTs to facilitate decision making in everyday practice. Further research is expected to shed light on the exact timing and optimal group of patients to discontinue treatment, and the rule of therapeutic drug monitoring at the time of drug cessation. However, it is recommended to make individual decisions; predictive factors of relapse, evidence of mucosal healing (including laboratory, endoscopic or radiological techniques) should be included in the risk/benefit analysis prior to therapy withdrawal.
AUTHORSHIP
Guarantor of the article: Patrícia Sarlós.
Author contributions: Conceptualisation, DD and PS; methodology, DD, PS and LH; selection of studies and data extraction, DD and PP; investigation, DD and PS; statistical analysis, LH; risk of bias assessment and certainty of evidence assessment with GRADE approach, DD and SzK; writing‐original draft preparation, DD and PS; visualisation, DD, PS and LH; review and editing, LH, SzK, ZsSz, AP and BE; resources and funding acquisition, PH and PS.
All authors approved the final version of the manuscript.
Personal and funding interests: This study was founed in part by European Union (European Regional Development Fund) within the framework of Programme Széchenyi 2020 [GINOP‐2.3.2‐15‐2016‐00048‐STAY ALIVE], and by Human Resources Development Operational Programme Grant [EFOP 3.6.2‐16‐2017‐00006‐LIVE LONGER], and by National Research, Development and Innovation Office [FK132834 to PS].
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Table S1
Table S2
Table S3
Table S4
ACKNOWLEDGEMENTS
Declaration of personal interests: None.
Dohos D, Hanák L, Szakács Z, et al. Systematic review with meta‐analysis: the effects of immunomodulator or biological withdrawal from mono‐ or combination therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2020;53:220–233. 10.1111/apt.16182
As part of AP&T’s peer‐review process, a technical check of this meta‐analysis was performed by Dr Y Yuan. The Handling Editor for this article was Professor Jonathan Rhodes, and it was accepted for publication after full peer‐review.
Funding information
This study was funded by the National Research, Development and Innovation Office (FK 132834 to PS) and “GINOP‐2.3.2‐15‐2016‐00048 ‐ STAY ALIVE” co‐financed by the European Union (European Regional Development Fund) within the framework of Programme Széchenyi 2020, and by Human Resources Development Operational Programme Grant, Grant Number: EFOP 3.6.2‐16‐2017‐00006 – LIVE LONGER, which is co‐financed by the European Union (European Regional Development Fund) within the framework of Programme Széchenyi 2020.
[Correction added on February 16, 2021, after first online publication: The copyright line was changed.]
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Crohn's Disease. Am Fam Physician 2018;98:Online. [PubMed] [Google Scholar]
- 2. Peyrin‐Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. Long‐term complications, extraintestinal manifestations, and mortality in adult Crohn's disease in population‐based cohorts. Inflamm Bowel Dis. 2011;17:471‐478. [DOI] [PubMed] [Google Scholar]
- 3. Dignass A, Eliakim R, Magro F, et al. Second European evidence‐based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965‐990. [DOI] [PubMed] [Google Scholar]
- 4. Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta‐analysis of population‐based studies. Gastroenterology. 2013;145:996‐1006. [DOI] [PubMed] [Google Scholar]
- 5. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn's disease: medical treatment. J Crohns Colitis. 2020;14:4‐22. [DOI] [PubMed] [Google Scholar]
- 6. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence‐based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11:769‐784. [DOI] [PubMed] [Google Scholar]
- 7. Fredericks E, Watermeyer G. De‐escalation of biological therapy in inflammatory bowel disease: benefits and risks. South Afr Med J. 2019;109:745‐749. [DOI] [PubMed] [Google Scholar]
- 8. Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol. 2018;113:481‐517. [DOI] [PubMed] [Google Scholar]
- 9. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative Colitis in Adults. The American journal of gastroenterology. 2019;114(3):384‐413. [DOI] [PubMed] [Google Scholar]
- 10. D'Haens G. Risks and benefits of biologic therapy for inflammatory bowel diseases. Gut. 2007;56:725‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet (London, England). 2009;374:1617‐1625. [DOI] [PubMed] [Google Scholar]
- 12. Peyrin–Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141:1621‐1628.e5. [DOI] [PubMed] [Google Scholar]
- 13. Lopez A, Mounier M, Bouvier A‐M, et al. Increased risk of acute myeloid leukemias and myelodysplastic syndromes in patients who received thiopurine treatment for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12:1324‐1329. [DOI] [PubMed] [Google Scholar]
- 14. Bourrier A, Carrat F, Colombel J‐F, et al. Excess risk of urinary tract cancers in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Aliment Pharmacol Ther. 2016;43:252‐261. [DOI] [PubMed] [Google Scholar]
- 15. Doherty G, Katsanos KH, Burisch J, et al. European Crohn's and Colitis organisation topical review on treatment withdrawal ['exit strategies'] in inflammatory bowel disease. J Crohns Colitis. 2018;12:17‐31. [DOI] [PubMed] [Google Scholar]
- 16. Bouhnik Y, Scemama G, Taï R, et al. Long‐term follow‐up of patients with Crohn's disease treated with azathioprine or 6‐mercaptopurine. Lancet (London, England). 1996;347:215‐219. [DOI] [PubMed] [Google Scholar]
- 17. Lémann M, Mary J‐Y, Colombel J‐F, et al. A randomized, double‐blind, controlled withdrawal trial in Crohn's disease patients in long‐term remission on azathioprine. Gastroenterology. 2005;128:1812‐1818. [DOI] [PubMed] [Google Scholar]
- 18. O'Donoghue DP, Dawson AM, Powell‐Tuck J, Bown RL, Lennard‐Jones JE. Double‐blind withdrawal trial of azathioprine as maintenance treatment for Crohn's disease. Lancet (London, England). 1978;2:955‐957. [DOI] [PubMed] [Google Scholar]
- 19. Wenzl HH, Primas C, Novacek G, et al. Withdrawal of long‐term maintenance treatment with azathioprine tends to increase relapse risk in patients with Crohn's disease. Dig Dis Sci. 2015;60:1414‐1423. [DOI] [PubMed] [Google Scholar]
- 20. Vilien M, Dahlerup JF, Munck LK, Nørregaard P, Grønbaek K, Fallingborg J. Randomized controlled azathioprine withdrawal after more than two years treatment in Crohn's disease: increased relapse rate the following year. Aliment Pharmacol Ther. 2004;19:1147‐1152. [DOI] [PubMed] [Google Scholar]
- 21. Kim PS, Zlatanic J, Korelitz BI, Gleim GW. Optimum duration of treatment with 6‐mercaptopurine for Crohn's disease. Am J Gastroenterol. 1999;94:3254‐3257. [DOI] [PubMed] [Google Scholar]
- 22. Feagan BG, Fedorak RN, Irvine EJ, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn's disease. North American Crohn's Study Group Investigators. N Engl J Med. 2000;342:1627‐1632. [DOI] [PubMed] [Google Scholar]
- 23. Candy S, Wright J, Gerber M, Adams G, Gerig M, Goodman R. A controlled double blind study of azathioprine in the management of Crohn's disease. Gut. 1995;37:674‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sokol H, Seksik P, Nion‐Larmurier I, et al. When can we stop azathioprine in Crohn's disease patients in long‐term remission? Gastroenterology. 2009;136(5):A‐189. [Google Scholar]
- 25. Hawthorne AB, Logan RF, Hawkey CJ, et al. Randomised controlled trial of azathioprine withdrawal in ulcerative colitis. BMJ (Clinical research ed). 1992;305:20‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lobel EZ, Korelitz BI, Xuereb MA, Panagopoulos G. A search for the optimal duration of treatment with 6‐mercaptopurine for ulcerative colitis. Am J Gastroenterol. 2004;99:462‐465. [DOI] [PubMed] [Google Scholar]
- 27. Van Assche G, Magdelaine–Beuzelin C, D'Haens G, et al. Withdrawal of immunosuppression in Crohn's disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology. 2008;134:1861‐1868. [DOI] [PubMed] [Google Scholar]
- 28. Kierkuś J, Iwańczak B, Wegner A, et al. Monotherapy with infliximab versus combination therapy in the maintenance of clinical remission in children with moderate to severe Crohn disease. J Pediatr Gastroenterol Nutr. 2015;60:580‐585. [DOI] [PubMed] [Google Scholar]
- 29. Hisamatsu T, Kato S, Kunisaki R, et al. Withdrawal of thiopurines in Crohn's disease treated with scheduled adalimumab maintenance: a prospective randomised clinical trial (DIAMOND2). J Gastroenterol. 2019;54:860‐870. [DOI] [PubMed] [Google Scholar]
- 30. Gisbert JP, Marín AC, Chaparro M. The risk of relapse after anti‐TNF discontinuation in inflammatory bowel disease: systematic review and meta‐analysis. The American journal of gastroenterology. 2016;111:632‐647. [DOI] [PubMed] [Google Scholar]
- 31. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Group IEREW . Guideline for good clinical practice E6 (R2). November 2016. https://www.ich.org/products/guidelines/efficacy/article/efficacy‐guidelines.html
- 33. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 34. Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomized studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schünemann H, Brozek J, Guyatt G, Oxman A, et al.Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using The GRADE Approach. 2013. https://gdt.gradepro.org/app/handbook/handbook.html#h.svwngs6pm0f2
- 36. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177‐188. [DOI] [PubMed] [Google Scholar]
- 37. Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta‐analyses In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Chapter 10 (www.training.cochrane.org/handbook) [Google Scholar]
- 38. Chai‐Adisaksopha C, Thorlund K, Iorio A. Interpreting trial sequential analysis. Transfusion. 2016;56:2918‐2922. [DOI] [PubMed] [Google Scholar]
- 39. Kobayashi T, Motoya S, Nakamura S, et al. The first prospective, multicentre, randomized controlled trial on discontinuation of maintenance infliximab in ulcerative colitis in remission: endoscopic normalization does not guarantee successful discontinuation. Gastroneterology. 2020;158:SP1.S‐136. [Google Scholar]
- 40. Wynands J, Belbouab R, Candon S, et al. 12‐month follow‐up after successful infliximab therapy in pediatric crohn disease. J Pediatr Gastroenterol Nutr. 2008;46:293‐298. [DOI] [PubMed] [Google Scholar]
- 41. Chauvin A, Le Thuaut A, Belhassan M, et al. Infliximab as a bridge to remission maintained by antimetabolite therapy in Crohn's disease: a retrospective study. Dig Liver Dis. 2014;46:695‐700. [DOI] [PubMed] [Google Scholar]
- 42. Choi GJ, Park DI, Park JH, et al. Withdrawal of azathioprine in luminal Crohn's disease treated with infl iximab maintenance therapy: a retrospective case‐control study. J Gastroenterol Hepatol. 2010;25:A86. [Google Scholar]
- 43. Fiorino G, Cortes PN, Ellul P, et al. Discontinuation of infliximab in patients with ulcerative colitis is associated with increased risk of relapse: a multinational retrospective cohort study. Clin Gastroenterol Hepatol. 2016;14:1426‐1432.e1. [DOI] [PubMed] [Google Scholar]
- 44. Roblin X, Boschetti G, Williet N, et al. Azathioprine dose reduction in inflammatory bowel disease patients on combination therapy: an open‐label, prospective and randomised clinical trial. Aliment Pharmacol Ther. 2017;46:142‐149. [DOI] [PubMed] [Google Scholar]
- 45. Yerushalmy‐Feler A, Cohen S. Risk factors for disease relapse after stepping down from combination to anti‐TNF monotherapy in children with IBD. J Crohns Colitis. 2018;12:S455. [Google Scholar]
- 46. Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70(3):439‐444. [PubMed] [Google Scholar]
- 47. Zittan E, Kabakchiev B, Kelly OB, et al. Development of the Harvey‐Bradshaw Index‐pro (HBI‐PRO) score to assess endoscopic disease activity in Crohn's disease. J Crohns Colitis. 2017;11:543‐548. [DOI] [PubMed] [Google Scholar]
- 48. Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660‐1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Baron JH, Connell AM, Lennard‐Jones JE. Variation between observers in describing mucosal appearances in proctocolitis. BMJ. 1964;1:89‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ (Clinical research ed). 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 51. Boyapati RK, Torres J, Palmela C, et al. Withdrawal of immunosuppressant or biologic therapy for patients with quiescent Crohn's disease. Cochrane Database Syst Rev. 2018;5:Cd012540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Torres J, Boyapati RK, Kennedy NA, Louis E, Colombel JF, Satsangi J. Systematic review of effects of withdrawal of immunomodulators or biologic agents from patients with inflammatory bowel disease. Gastroenterology. 2015;149:1716‐1730. [DOI] [PubMed] [Google Scholar]
- 53. Kennedy NA, Warner B, Johnston EL, et al. Relapse after withdrawal from anti‐TNF therapy for inflammatory bowel disease: an observational study, plus systematic review and meta‐analysis. Aliment Pharmacol Ther. 2016;43:910‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vermeire S, Noman M, Van Assche G, Baert F, D'Haens G, Rutgeerts P. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn's disease. Gut. 2007;56:1226‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kennedy NA, Heap GA, Green HD, et al. Predictors of anti‐TNF treatment failure in anti‐TNF‐naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4:341‐353. [DOI] [PubMed] [Google Scholar]
- 56. Chalhoub JM, Rimmani HH, Gumaste VV, Sharara AI. Systematic review and meta‐analysis: adalimumab monotherapy versus combination therapy with immunomodulators for induction and maintenance of remission and response in patients with Crohn's disease. Inflamm Bowel Dis. 2017;23:1316‐1327. [DOI] [PubMed] [Google Scholar]
- 57. Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long‐term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003;348:601‐608. [DOI] [PubMed] [Google Scholar]
- 58. Qiu Y, Mao R, Chen BL, et al. Effects of combination therapy with immunomodulators on trough levels and antibodies against tumor necrosis factor antagonists in patients with inflammatory bowel disease: a meta‐analysis. Clin Gastroenterol Hepatol. 2017;15:1359‐1372.e1356. [DOI] [PubMed] [Google Scholar]
- 59. Matsumoto T, Motoya S, Watanabe K, et al. Adalimumab monotherapy and a combination with azathioprine for Crohn's disease: a prospective, randomized trial. J Crohns Colitis. 2016;10:1259‐1266. [DOI] [PubMed] [Google Scholar]
- 60. Sandborn WJ, Rutgeerts P, Gasink C, et al. Long‐term efficacy and safety of ustekinumab for Crohn's disease through the second year of therapy. Aliment Pharmacol Ther. 2018;48:65‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711‐721. [DOI] [PubMed] [Google Scholar]
- 62. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699‐710. [DOI] [PubMed] [Google Scholar]
- 63. Steenholdt C, Molazahi A, Ainsworth MA, Brynskov J, Østergaard Thomsen O, Seidelin JB. Outcome after discontinuation of infliximab in patients with inflammatory bowel disease in clinical remission: an observational Danish single center study. Scand J Gastroenterol. 2012;47:518‐527. [DOI] [PubMed] [Google Scholar]
- 64. Chaparro MGD, Barreiro‐de Acosta M, Domènech E, et al. Anti‐tumour necrosis factor discontinuation in inflammatory bowel disease patients in remission: study protocol of a prospective, multicentre, randomized clinical trial. Ther Adv Gastroenterol. 2019;12:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stopping Biological Therapy in PCD Study. Study protocol in www.clinicaltrial.gov. https://clinicaltrials.gov/ct2/show/NCT04129723
- 66. A proSpective Randomized Controlled Trial comParing infliximAb‐antimetabolites Combination Therapy to Anti‐metabolites monotheRapy and Infliximab monothErapy in Crohn's Disease Patients in Sustained Steroid‐free Remission on Combination Therapy (SPARE). Study protocol in www.clinicaltril.gov. https://clinicaltrials.gov/ct2/show/NCT02177071
- 67. Gisbert JP, Marín AC, Chaparro M. Systematic review: factors associated with relapse of inflammatory bowel disease after discontinuation of anti‐TNF therapy. Aliment Pharmacol Ther. 2015;42:391‐405. [DOI] [PubMed] [Google Scholar]
- 68. Ben‐Horin S, Chowers Y, Ungar B, et al. Undetectable anti‐TNF drug levels in patients with long‐term remission predict successful drug withdrawal. Aliment Pharmacol Ther. 2015;42:356‐364. [DOI] [PubMed] [Google Scholar]
- 69. Baert F, Drobne D, Gils A, et al. Early trough levels and antibodies to infliximab predict safety and success of reinitiation of infliximab therapy. Clin Gastroenterol Hepatol. 2014;12(9):1474‐1481.e1472; quiz e1491. [DOI] [PubMed] [Google Scholar]
- 70. Mao R, Xiao YL, Gao X, et al. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta‐analysis of prospective studies. Inflamm Bowel Dis. 2012;18:1894‐1899. [DOI] [PubMed] [Google Scholar]
- 71. Molander P, Färkkilä M, Salminen K, et al. Outcome after discontinuation of TNFα‐blocking therapy in patients with inflammatory bowel disease in deep remission. Inflamm Bowel Dis. 2014;20:1021‐1028. [DOI] [PubMed] [Google Scholar]
- 72. Park S, Abdi T, Gentry M, Laine L. Histological disease activity as a predictor of clinical relapse among patients with ulcerative colitis: systematic review and meta‐analysis. Am J Gastroenterol. 2016;111:1692‐1701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Table S1
Table S2
Table S3
Table S4
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


