FIGURE 8.
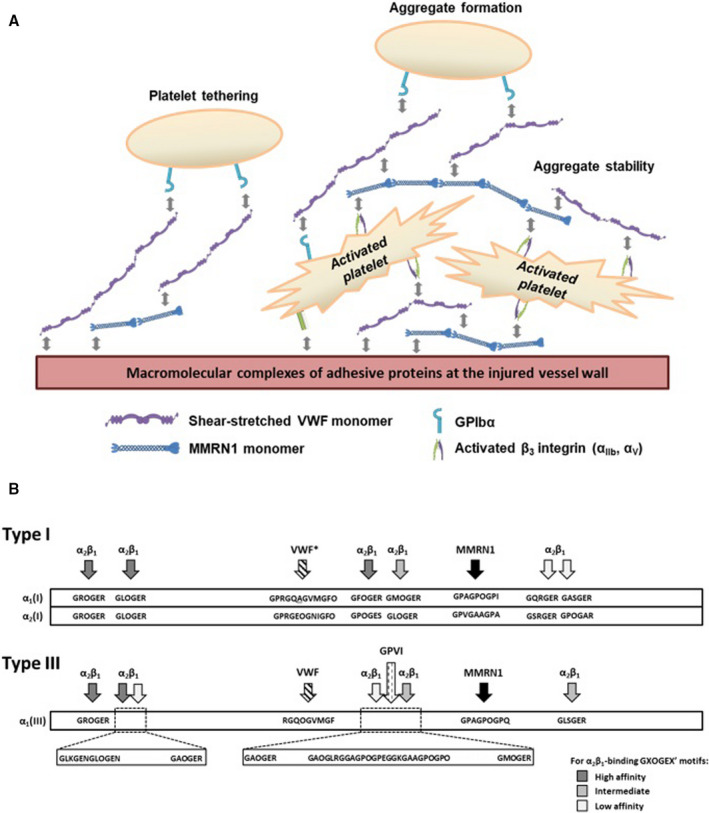
Proposed model of multimerin 1 (Mmrn1) functions in platelet adhesion and an updated model of the motifs in types I and III vessel wall fibrillar collagens that support platelet adhesion or activation. A, Proposed role of Mmrn1 in platelet adhesion. Following Mmrn1 release from platelets and endothelial cell storage granules, αIIbβ3, αvβ3, and other unidentified receptors mediate Mmrn1 binding to platelets. Mmrn1 binding to platelets increases the size and stability of platelet aggregates that are captured onto the macromolecular protein complexes that promote platelet–matrix and platelet–platelet interactions. Shear‐exposed and matrix bound von Willebrand factor (Vwf), fibrin(ogen), and fibronectin in these macromolecular protein complexes provide multiple binding sites for Mmrn1 attachment. Additionally, at sites of injury that expose blood to fibrillar collagen, Mmrn1 binds to GPAGPOGPX motifs to synergistically increase platelet adhesion to collagen beyond the adhesion supported by Vwf‐ and α2β1‐dependent mechanisms. B, Scale representation of the spatial arrangement of the functional sequences in the triple‐helical (COL) domain of human type I (top) and type III (bottom) collagen that support platelet adhesion or activation. *The α1‐α1‐α2 heterotrimer consisting of GPRGQAGVMGFO and GPRGEOGNIGFO in type I collagen has been verified to support VWF binding using heterotrimeric triple‐helical peptides. 72 GXOGER sequences that bind α2β1, the VWF‐binding sequence GPRGQOGVMGFO, and the GPVI‐binding sequence GAOGLRGAGPOGPEGGKGAAGPOGPO have been previously described elsewhere
