Figure 1.
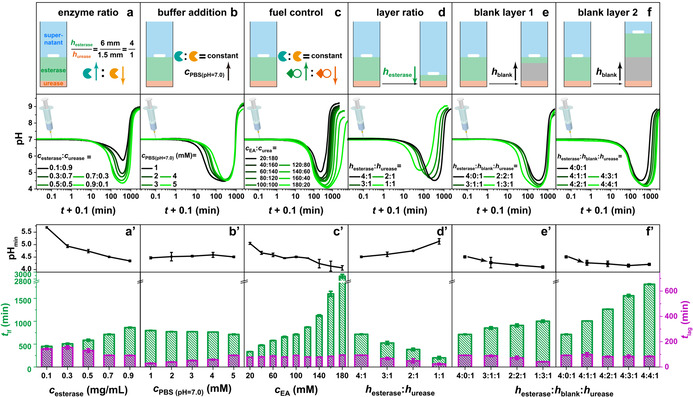
Programming the t lf, t lag and pHmin of transient acidic pH flips: a) Increasing the c esterase leads to a pronounced pHmin, longer t lf and shorter t lag. Conditions: h esterase:h urease=4:1, c EA=c urea=100 mM, c esterase + c urease=1.0 mg mL−1, and 5 mM PBS (pH 7.0; 0.5 mg mL−1 BSA; 20 °C). b) The t lag is controlled by the buffer concentration. Conditions: h esterase:h urease=4:1, 0.7 mg mL−1 esterase, 0.3 mg mL−1 urease, c EA=c urea=100 mM, 0.5 mg mL−1 BSA, and 20 °C. c) Adjusting the ratio of EA and urea results in a wide tuneability of t lf and pHmin. Conditions: h esterase:h urease=4:1, 0.7 mg mL−1 esterase, 0.3 mg mL−1 urease, c EA + c urea=200 mM, and 5 mM PBS (pH 7.0; 0.5 mg mL−1 BSA; 20 °C). d) The height ratio of esterase and urease layers regulates the pHmin, t lf and t lag. Conditions: 0.7 mg mL−1 esterase, 0.3 mg mL−1 urease, c EA=c urea=100 mM, and 5 mM PBS (pH 7.0; 0.5 mg mL−1 BSA; 20 °C). e) Effects of installing a blank hydrogel layer between esterase and urease layers on the expense of the esterase layer thickness. Conditions: 0.7 mg mL−1 esterase, 0.3 mg mL−1 urease, c EA=c urea=100 mM, and 5 mM PBS (pH 7.0; 0.5 mg mL−1 BSA; 20 °C). f) Effects of installing a blank layer while maintaining esterase and urease layer thickness. Conditions: 0.7 mg mL−1 esterase, 0.3 mg mL−1 urease, c EA=c urea=100 mM, and 5 mM PBS (pH 7.0; 0.5 mg mL−1 BSA; 20 °C). (a′–f′) The corresponding pHmin, t lag and t lf of the transient acidic pH flips in (a–f). All data are an average of three measurements, detailed experimental conditions are listed in Table S2.
