Figure 1.
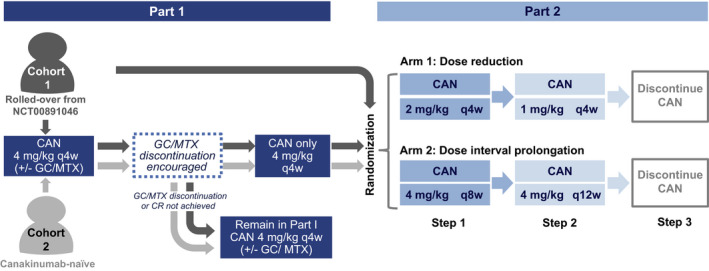
Flow chart of the study design. Cohort 1 comprised patients with systemic juvenile idiopathic arthritis in complete clinical remission (CR) at the last visit of a previous long‐term extension trial of canakinumab (CAN) (ClinicalTrials.gov identifier: NCT00891046) (8). Patients in cohort 1 who were already receiving canakinumab monotherapy could enter directly into part 2 of the study. Cohort 2 comprised newly recruited patients with active disease at baseline. Patients in whom clinical remission was achieved with canakinumab monotherapy for at least 4 weeks in part 1 entered part 2 of the study (as long as the randomization period was still open). Patients in whom these criteria were not met, or in whom criteria were met after randomization was closed, remained in part 1 until study end. Randomization was only to achieve balance between the treatment arms as no comparison between the cohorts was planned. GC = glucocorticoid; MTX = methotrexate; q4w = every 4 weeks.
