Figure 2.
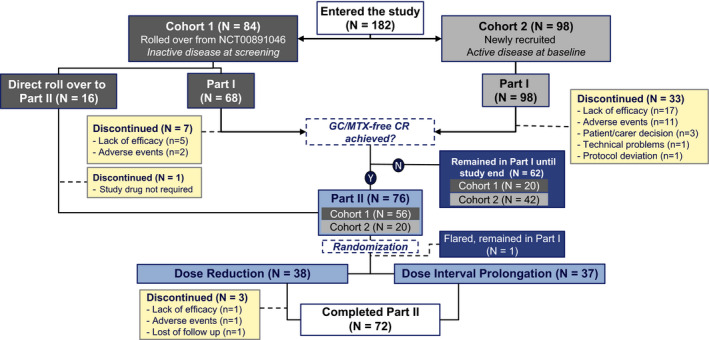
Flow chart of the study population. In total, 182 individuals entered the study, with 44 discontinuing the study and 138 completing it. Sixty‐three patients remained in part 1 until study end, and 72 patients completed part 2. After reaching the predefined population of 76 patients for part 2 (patients in whom clinical remission [CR] with canakinumab monotherapy was achieved without glucocorticoids [GCs] or methotrexate [MTX]), randomization enrollment was closed, and patients in whom clinical remission was achieved with canakinumab monotherapy after this time remained in part 1 of the study. Among the 62 patients who were not randomized and remained in part 1 until study end, protocol deviations were reported in 4 patients who were not randomized but progressed to the canakinumab tapering treatment arm, with 1 patient enrolled directly from the long‐term extension trial of canakinumab (ClinicalTrials.gov identifier: NCT00891046) (8) and receiving 1 dose according to the dose reduction scheme before discontinuing the study and 3 additional patients in part 1 progressing to the dose interval prolongation treatment arm. These patients were not included in the efficacy analyses of part 2. Y = yes; N = no.
