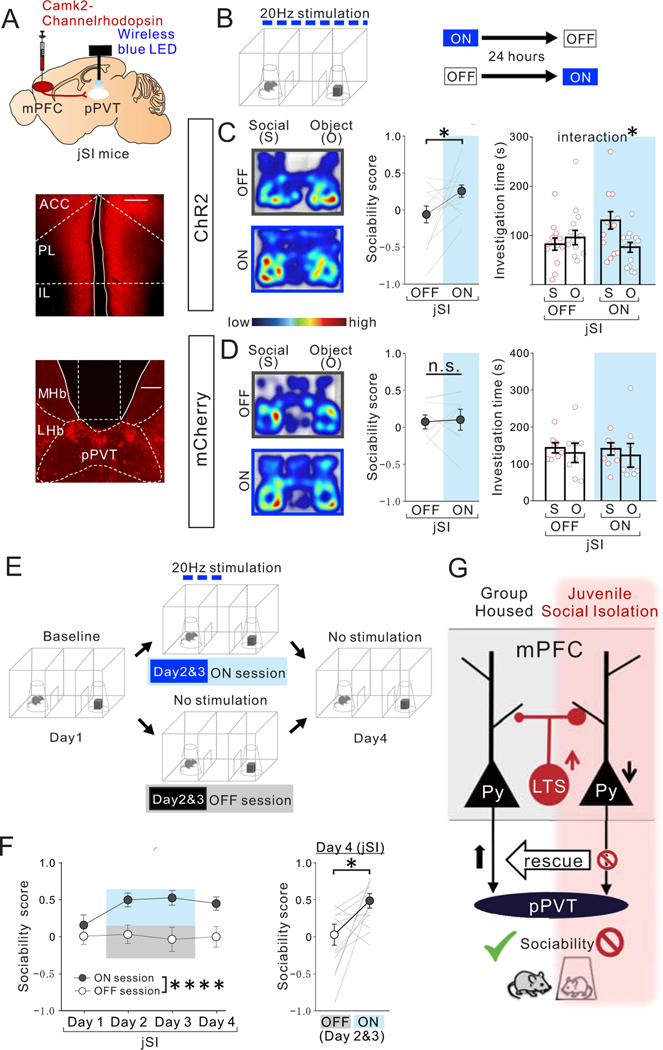
Figure. 8. Optogenetic activation of mPFC->pPVT projection terminals rescues sociability deficits in adult jSI mice.
(A) (top) AAV encoding Channelrhodopshin ChR2 under the CamK2 promotor was injected into the mPFC and a wireless blue LED was inserted above the pPVT of adult jSI mice. Representative images of mPFC (middle) and pPVT (bottom) show selective transduction of ChR2 in injection areas in the mPFC, and at projection target areas in the pPVT where optic fibers are located (middle; scale bar = 600 μm, bottom; scale bar = 300 μm). Experimental images were obtained from 13 mice, three images per mouse for each area, with similar results obtained. (B) Adult jSI mice underwent the 3 chamber test for sociability with (ON) or without (OFF) light stimulation. ON and OFF sessions were counter-balanced with a 24-hour interval between each behavior test. (C) ChR2+ jSI mice receiving optogenetic stimulation showed increased sociability scores (two tailed paired t-test, t12=2.284, *P=0.041, n=13 biologically independent jSI mice) and increased social interaction (two-way RM ANOVA, light (ON/OFF) × stimulus (social/object) interaction F1,24 = 5.064, *P=0.034, effect of light F1,24 = 1.224, P=0.280, effect of stimulus F1,24 = 1.865, P=0.185, n=13 biologically independent jSI mice). (D) Control mCherry+ mice showed no difference in sociability score (two tailed paired t-test, t7=0.258, P=0.804, n=8 biologically independent jSI mice) and investigation time (two-way RM ANOVA, light (ON/OFF) × stimulus (social/object) interaction F1,14 = 0.007, P=0.936, effect of light F1,14 = 0.082, P=0.779, effect of stimulus F1,14 = 0.457, P=0.510, n=6 biologically independent jSI mice). (E) Experimental paradigm to examine how long the effect of optogenetic stimulation of mPFC->pPVT projection persists. Baseline day (Day1): mice (12 mice) explored the 3-chamber arena with novel mouse and novel object corrals for 20 mins. (Days 2 & 3): Mice underwent the same 3-chamber test as on Day 1, except mPFC->pPVT circuits were stimulated whenever test mice entered the social interaction zone surrounding a wire corral and the session was extended to 30 min. A probe test day (Day4): mice underwent the 3-chamber test in the absence of optogenetic stimulation for 20 mins. (F) (Left) Sociability scores from Day1 to Day4 (first 20 mins) show a significant sustained effect of light stimulation on Day 2 and 3 (two-way RM ANOVA, light (ON/OFF) × day (Day1/Day2/Day3/Day4) interaction F3,66 = 1.172, P=0.327, effect of light F1,22 = 45.940, ****P=0.823×10−6, effect of day F2.054, 45.18 = 1.012, P=0.373, n=12 biologically independent jSI mice). (Right) On Day 4, recovery of sociability persisted in the absence of light stimulation (two tailed paired t-test, t11=2.654, *P=0.022, n=12 biologically independent jSI mice). Data in C, D, F are presented as mean +/− s.e.m. (G) Summary Scheme: Activation of mPFC->pPVT projection neurons is essential for normal sociability in adult GH mice. However, these neurons show decreased intrinsic excitability and an increased inhibitory input drive from mPFC LTS-SST interneurons when deprived of juvenile social experience, a manipulation that leads to decreased social interaction in adulthood. Decreased social interaction can be induced in GH animals by inhibiting mPFC->pPVT projection or activating mPFC LTS-SST interneurons in adulthood and sociability of jSI mice can be rescued by increasing PFC->pPVT projection neuron activity.