Abstract
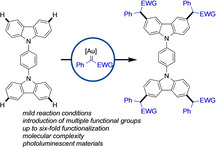
Herein we describe a multiple C−H functionalization reaction of carbazole heterocycles with diazoalkanes. We show that gold catalysts play a distinct role in enabling a multiple C−H functionalization reaction to introduce up to six carbene fragments onto molecules containing multiple carbazole units or to link multiple carbazole units into a single molecule. A one‐pot stepwise approach enables the introduction of two different carbene fragments to allow orthogonal deprotection and straightforward derivatization.
Keywords: carbazoles, carbenes, diazoalkanes, gold, multi C−H functionalization
Paved with gold: We describe a multiple C−H functionalization reaction of carbazole heterocycles with diazoalkanes. We show that gold catalysts play a distinct role in enabling a multiple C−H functionalization reaction to introduce up to six carbene fragments onto molecules containing multiple carbazole units or to link multiple carbazole units into a single molecule. A one‐pot stepwise approach enables the introduction of two different carbene fragments to allow orthogonal deprotection and straightforward derivatization.
C−H functionalization reactions are a facile strategy to directly introduce new functional groups onto organic frameworks without the need for specific pre‐functionalization of the substrate molecule.[ 1 , 2 , 3 ] In this context, the use of metal‐catalyzed carbene transfer reactions for C−H functionalization has emerged as an important strategy to construct new C−C bonds. [2] The reactivity and site‐selectivity of the C−H functionalization reaction can be typically controlled by the choice of catalyst. Previous reports using precious metal catalysts such as RhII, [3] AuI, [4] PdII, [5] or others [6] have demonstrated the potential of this approach in the single functionalization of discrete C−H bonds (Scheme 1 a). To the best of our knowledge, the functionalization of multiple C−H bonds, in a highly site‐selective and controlled stepwise manner, has been studied only to a very limited extent. In this context, the Davies group reported on the double C−H functionalization of 1,5‐cyclooctadiene to access a new chiral family of COD ligands (Scheme 1 b). [7]
Scheme 1.
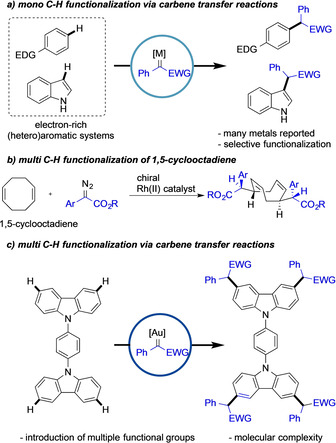
Mono and multi C−H functionalization reactions.
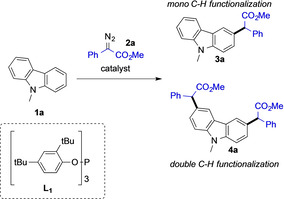
Based on our previous studies,[ 8 , 9 ] we hypothesized that double benzannellated heterocycles could undergo multiple consecutive site‐selective C−H functionalization reactions.[ 7 , 8a ] In this context, the carbazole heterocycle is particularly suited due to its two flanking benzenoid rings that can be subjected to C−H functionalization. [7] Moreover, the carbazole framework finds regular applications in materials’ chemistry [10] and drugs [11] and thus methods to introduce multiple functional groups in a streamlined fashion are demanded for further applications, such as the development of photoluminescent materials. In general, the C−H functionalization of electron‐rich arenes (Scheme 1 a), a class of compounds which carbazole belongs to, can occur in different positions and thus a chemoselective C−H functionalization is key to further address the challenge of multi C−H functionalization reactions. Recently, we have uncovered a highly selective C−H functionalization reaction of unprotected carbazoles using a AuI catalysts featuring high reactivity and reaction times of less than 15 minutes.[ 7 , 12 ] We believe that the efficiency and rapid catalyst turnover of AuI complexes were the key to achieve both site‐selectivity and reactivity in those reactions. Thus, such catalytic system can potentially also address the synthetic challenge of multi C−H functionalization reaction (Scheme 1 c).
We set out our investigations by first identifying a suitable catalyst to conduct multiple C−H functionalization reactions of N‐methyl carbazole 1 a with an excess of the diazoalkane reagent 2 a. Among the pool of organometallic complexes we examined, catalysts based on PdII, RhII or CuI unsurprisingly provided only non‐detectable to minor amounts of the double C−H functionalization product (Table 1, entries 1–4). Gratifyingly, we were met with initial success when testing AuI NHC complexes for the reaction (Table 1, entries 5 and 6). These catalysts led to the formation of the double C−H functionalized 4 a as the major reaction product, though only in moderate yield and with a substantial amount of the undesired monofunctionalized product 3 a. These results encouraged us to examine different mono‐ and bidentate phosphine ligands for the AuI catalyst. Among all phosphines tested, notable results were obtained using XPhos (Table 1, entry 7), which gave a good yield and a good selectivity towards the double functionalization product. However, the best results were achieved by using an AuI complex with phosphite‐derived ligand (L1) in diluted reaction mixture and a reaction time of 3 hours (Table 1, entry 15). Further investigations, regarding the counteranion, [13] solvent and reaction stoichiometry, did not improve the reaction outcomes (for details, please see Table S1 in the ESI).
Table 1.
Reaction optimization.
|
| |||
|---|---|---|---|
|
#[a] |
Catalyst |
Additive |
Yield [%] (3 a/4 a)[d] |
|
1 |
Pd(OAc)2 |
PPh3 |
11/n.d. |
|
2 |
Rh2(OAc)4 |
– |
51/17 |
|
3 |
Rh2(esp)2 |
– |
42/26 |
|
4 |
CuPF6(MeCN)4 |
2,2’‐bipyridine |
19/n.d. |
|
5 |
(IPr)AuCl |
AgSbF6 |
16/46 |
|
6 |
(IMes)AuCl |
AgSbF6 |
10/49 |
|
7 |
(XPhos)AuCl |
AgSbF6 |
8/69[e] |
|
8 |
(tBu‐XPhos)AuCl |
AgSbF6 |
23/50 |
|
9 |
(JohnPhos)AuCl |
AgSbF6 |
13/61 |
|
10 |
(tBu3P)AuNTf2 |
– |
42/16 |
|
11 |
dppf(AuCl)2 |
AgSbF6 |
41/31 |
|
12 |
(L1)AuCl |
AgSbF6 |
<5/76[e] |
|
13 |
(L1)AuCl |
AgBF4 |
7/ 32 |
|
14 |
(L1)AuCl |
AgPF6 |
17/ 44 |
|
15[b] |
(L1)AuCl |
AgSbF6 |
<5/84[e] |
|
16[c] |
(L1)AuCl |
AgSbF6 |
<5/67[e] |
[a] Reaction conditions: 0.2 mmol 1 a, 5 mol % catalysts, 6.0 mol % additive were dissolved in 1.5 mL dry CH2Cl2. 2 a (0.8 mmol, 4.0 equiv) was dissolved in 0.5 mL of dry CH2Cl2 and added to the reaction mixture over 60 min. The reaction mixture was stirred for an additional 2 h at RT under argon. [b] 4 mL of dry CH2Cl2 was used. [c] With 1.6 mol % catalyst, 2.0 mol % additive on 0.6 mmol scale in 4.5 mL CH2Cl2. [d] Yield determined by 1H NMR integration, 0.2 mmol mesitylene was used as an internal standard; n.d.=not detected. [e] Yield of isolated product. L1=tris(2,4‐di‐tert‐butylphenyl)phosphite.
Having established the optimal conditions to selectively introduce two carbene fragments onto N‐methyl carbazole 1 a, we decided to explore the scope of this reaction with different carbazole heterocycles. N‐alkyl carbazoles (1 a–g) delivered the corresponding double C−H functionalized products (4 a–g) in good to high yields. 9‐(2‐Methylallyl)‐9H‐carbazole 1 f gave the product 4 f in decreased yield. N‐aryl carbazoles (1 h–k) underwent a similar double functionalization reaction and the corresponding products (4 h–k) were isolated in good to high yields. No by‐product formation arising from C−H functionalization of the N‐phenyl group was detected for 4 h or its analogues (4 i–4 k). This is a surprising observation and might be related to the aromatic character of the carbazole that decreases the nucleophilicity of the N‐phenyl group.
To probe the general feasibility of multi C−H functionalization of three different aromatic rings, we studied the reaction of triphenylamine with different stoichiometries of methyl phenyldiazoacetate 2 a. Interestingly, by only employing different stoichiometry, we could observe smooth mono (5 a), double (5 b), or triple (5 c) C−H functionalization of triphenylamine under otherwise identical reaction conditions (Scheme 2). These results are clear evidence for the applicability of our newly developed protocol to multi C−H functionalization of electron‐rich arenes in a highly selective and controlled manner.
Scheme 2.
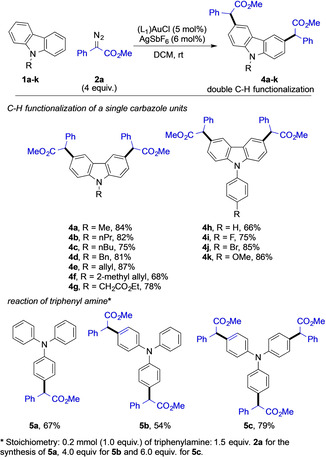
Double C−H functionalization reactions of carbazole and reaction with triphenyl amine. Isolated yields are shown.
In additional experiments, we probed a stepwise protocol (Scheme 3 a) to functionalize C−H bonds with different carbenes in one pot. For this purpose, methyl phenyldiazoacetate 2 a was added over 30 minutes to a mixture of carbazole 1 a and catalyst, let reacted for 30 minutes before the subsequent addition of a tert‐butyl phenyldiazoacetate 2 b over another 30 minutes. This approach gave the double functionalized carbazole 4 l bearing two orthogonally protected ester groups in comparable yield as in the case of the direct double functionalization with a single diazoalkane (Scheme 2, 4 a). In a similar fashion, N‐phenyl carbazole could be decorated with two different diazoalkanes and the product 4 m was obtained in high yield. This strategy thus enables to selectively introduce two new functional groups onto the carbazole scaffold with high selectivity and efficiency, further enhancing the applicability of our protocol.
Scheme 3.
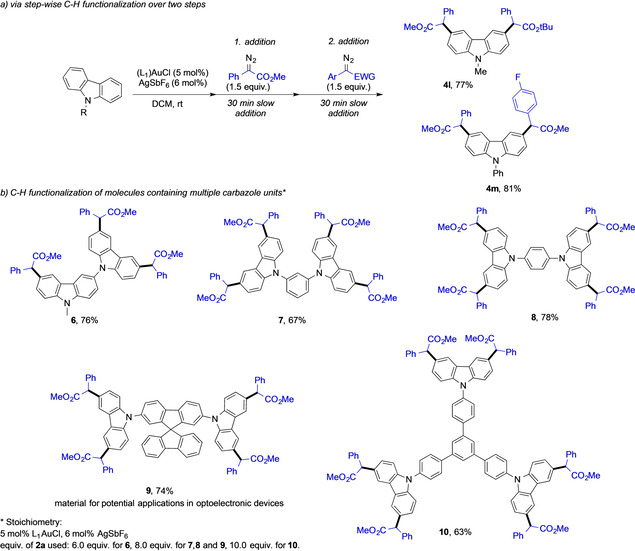
Stepwise protocol for the introduction of two different carbenes and multi C−H functionalization. Isolated yields are shown.
To further explore this concept, we subsequently investigated the C−H functionalization reaction of molecules containing multiple carbazole moieties (Scheme 3 b). Three carbene fragments could be transferred onto a 3,9′‐bicarbazole—one onto each reactive C−H bond of the carbazole skeleton framework—affording 6 in relatively high yield. When introducing different aromatic linkers between different carbazole units, C−H functionalization occurred in all activated positions of each carbazole unit. Arene‐linked dicarbazoles readily underwent four‐fold C−H functionalization using 8.0 equivalents of methyl phenyldiazoacetate 2 a to give 7, 8, and 9 in excellent yields for such type of transformations. When studying the terphenyl‐linked tricarbazole and 10.0 equivalents of methyl phenyldiazoacetate 2 a, even a six‐fold C−H functionalization reaction could be achieved to provide 10 in a pleasing yield of 63 %. An important point to be noted here is that all of these reactions in Scheme 3 were conducted using 5 mol % of the AuI catalyst and reaction times of only 3 hours.
After establishing conditions to introduce multiple carbene fragments onto one carbazole unit, we next aimed at demonstrating the connection of multiple carbazole units (Scheme 4) by gold‐catalyzed carbene transfer reactions of bis‐diazoalkane 11. This reagent should ideally serve as a dual carbene linchpin reagent and allow the linkage of two identical fragments on both carbene units. We thus investigated the reaction of 1.0 equivalent of the linchpin reagent 11 with an excess of N‐methyl carbazole 1 a, yet only an unidentifiable mixture of products was obtained, which can be rationalized by uncontrolled polymerization reactions. To prevent the polymerization of both reaction partners, the linchpin reagent 11 was then slowly added via syringe pump over one hour, which then gave access to the dicarbazole 12 in good yield (Scheme 4). Relevant to this type of linchpin reactivity, when we switched from methyl phenyldiazoacetate 2 a to (1‐diazo‐2,2,2‐trifluoroethyl)benzene (13) in reaction with N‐methyl carbazole 1 a, we observed a surprising reaction. Compound 13 unexpectedly underwent a double C−H functionalization reaction with two carbazole molecules to give the short‐linked compound 14 in good yields. This fluorinated diazoalkane thus acts as an equivalent of bis‐diazoalkane 11 in the role of a linchpin reagent to link two carbazole molecules into one framework, though presumably via a different mechanism. We hypothesized that this reaction occurred via formation of a benzylic cation intermediate (15), followed by a Friedel–Crafts type electrophilic substitution reaction to a second carbazole molecule. Compound 15 can also be transformed into 14 via an electrophilic coupling reaction with a gold alkyl complex intermediate (15’). The formation of 15 or 15’ has been documented in literature before. [14]
Scheme 4.
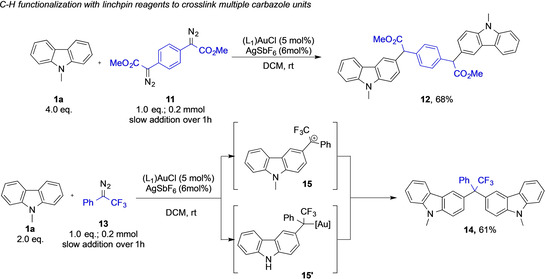
Connection of multiple carbazole units via linchpin reagents. Isolated yields are shown.
In summary, we report on the multi C−H functionalization reaction of aryldiazoacetates with carbazole heterocycles. While typical carbene transfer catalysts give only diminutive amounts of the multi‐functionalization product, it was shown that gold catalyst exhibits a distinct role in this reaction and enables the introduction of up to six carbene fragments onto (poly)carbazole frameworks. The application of linchpin reagents, bearing two carbene precursors, allows the linkage of two carbazole fragments and opens up new pathways toward polycarbazoles via C−H functionalization of simple and readily accessible building blocks.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
R.M.K. thanks the German Science Foundation and Dean's Seed Fund of RWTH Aachen Foundation for financial support. The authors acknowledge the DAAD‐PPP and Universities Australia for sponsoring the travels associated with this work. The authors thank the Australian Research Council for funding (FT180100260 to T.V.N. and DP200100063 to T.V.N. and R.M.K.). Open access funding enabled and organized by Projekt DEAL.
S. Jana, C. Empel, T. V. Nguyen, R. M. Koenigs, Chem. Eur. J. 2021, 27, 2628.
Contributor Information
Dr. Thanh Vinh Nguyen, Email: t.v.nguyen@unsw.edu.au.
Prof. Dr. Rene M. Koenigs, Email: rene.koenigs@rwth-aachen.de.
References
- 1.
- 1a. Girard S. A., Knauber T., Li C.-J., Angew. Chem. Int. Ed. 2014, 53, 74–100; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 76–103; [Google Scholar]
- 1b. Gensch T., Hopkinson M. N., Glorius F., Wencel-Delord J., Chem. Soc. Rev. 2016, 45, 2900–2936; [DOI] [PubMed] [Google Scholar]
- 1c. Moselage M., Lie J., Ackermann L., ACS Catal. 2016, 6, 498–525; [Google Scholar]
- 1d. Shang R., Ilies L., Nakamura E., Chem. Rev. 2017, 117, 9086–9139; [DOI] [PubMed] [Google Scholar]
- 1e. Yamaguchi J., Yamaguchi A. D., Itami K., Angew. Chem. Int. Ed. 2012, 51, 8960–9009; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 9092–9142. [Google Scholar]
- 2.
- 2a. Davies H. M. L., Manning J. R., Nature 2008, 451, 417–424; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2b. Doyle M. P., Duffy R., Ratnikov M., Zhou Z., Chem. Rev. 2010, 110, 704–724; [DOI] [PubMed] [Google Scholar]
- 2c. Empel C., Jana S., Koenigs R. M., Molecules 2020, 25, 880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.
- 3a. DeAngelis A., Shurtleff V. W., Dmitrenko O., Fox J. M., J. Am. Chem. Soc. 2011, 133, 1650–1653; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3b. Chan W.-W., Lo S.-F., Zhou Z., Yu W.-Y., J. Am. Chem. Soc. 2012, 134, 13565–13568; [DOI] [PubMed] [Google Scholar]
- 3c. Liao K., Liu W., Niemeyer Z. L., Ren Z., Bacsa J., Musaev D. G., Sigman M. S., Davies H. M. L., ACS Catal. 2018, 8, 678–682. [Google Scholar]
- 4.For reviews:
- 4a. Hashmi A. S. K., Salathé R., Frost T. M., Schwarz L., Choi J.-H., Appl. Catal. A 2005, 291, 238–246; [Google Scholar]
- 4b. Liu L., Zhang J., Chem. Soc. Rev. 2016, 45, 506–516; [DOI] [PubMed] [Google Scholar]
- 4c. Fructos M. R., Diaz-Requejo M. M., Perez P. J., Chem. Commun. 2016, 52, 7326–7335; [DOI] [PubMed] [Google Scholar]
- 4d. Ma B., Liu L., Zhang J., Asian J. Org. Chem. 2018, 7, 2015–2025; [Google Scholar]
- 4e. Zhao X., Rudolph M., Hashmi A. S. K., Chem. Commun. 2019, 55, 12127–12135; [DOI] [PubMed] [Google Scholar]
- 4f. Hashmi A. S. K., Acc. Chem. Res. 2014, 47, 864–876; [DOI] [PubMed] [Google Scholar]
- 4g. Braun I., Asiri A. M., Hashmi A. S. K., ACS Catal. 2013, 3, 1902–1907. [Google Scholar]
- 5.
- 5a. Yang Z., Möller M., Koenigs R. M., Angew. Chem. Int. Ed. 2020, 59, 5572–5576; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 5620–5624; [Google Scholar]
- 5b. Arredondo V., Hiew S. C., Gutman E. S., Premachandra I. D. U. A., van Vranken D., Angew. Chem. Int. Ed. 2017, 56, 4156–4159; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 4220–4223. [Google Scholar]
- 6.
- 6a. Hock K. J., Knorrscheidt A., Hommelsheim R., Ho J., Weissenborn M. J., Koenigs R. M., Angew. Chem. Int. Ed. 2019, 58, 3630–3634; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 3669–3673; [Google Scholar]
- 6b. Vargas D. A., Tinoco A., Tyagi V., Fasan R., Angew. Chem. Int. Ed. 2018, 57, 9911–9915; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 10059–10063; [Google Scholar]
- 6c. Ciszewski L. W., Durka J., Gryko D., Org. Lett. 2019, 21, 7028–7032; [DOI] [PubMed] [Google Scholar]
- 6d. Delgado-Rebollo M., Prieto A., Pérez P. J., ChemCatChem 2014, 6, 2047–2052; [Google Scholar]
- 6e. Gnad F., Poleschak M., Reiser O., Tetrahedron Lett. 2004, 45, 4277–4280. [Google Scholar]
- 7. Zhang B., Hollerbach M. R., Blakey S. R., Davies H. M. L., Org. Lett. 2019, 21, 9864–9868. [DOI] [PubMed] [Google Scholar]
- 8.
- 8a. Jana S., Empel C., Pei C., Aseeva P., Nguyen T. V., Koenigs R. M., ACS Catal. 2020, 10, 9925–9931; [Google Scholar]
- 8b. Tran U. P. N., Hommelsheim R., Yang Z., Empel C., Hock K. J., Nguyen T. V., Koenigs R. M., Chem. Eur. J. 2020, 26, 1254–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.
- 9a. Empel C., Koenigs R. M., Synlett 2019, 30, 1929–1934; [Google Scholar]
- 9b. Pei C., Yang Z., Koenigs R. M., Org. Lett. 2020, 22, 7300–7304; [DOI] [PubMed] [Google Scholar]
- 9c. Jana S., Yang Z., Pei C., Xu X., Koenigs R. M., Chem. Sci. 2019, 10, 10129–10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.
- 10a. Schmidt A. W., Reddy K. R., Knoelker H.-J., Chem. Rev. 2012, 112, 3193–3328; [DOI] [PubMed] [Google Scholar]
- 10b. Lia J., Grimsdale A. C., Chem. Soc. Rev. 2010, 39, 2399–2410. [DOI] [PubMed] [Google Scholar]
- 11.
- 11a. Mickle T., Guenther S., Mickle C., Chi G., Kanski J., Martin A. K., Bera B. (Kempharm, Inc.), Int. PCT Pub. No. WO2011002995 A1, 2010;
- 11b. Nasiri S., Cekaviciute M., Simokaitiene J., Petrauskaite A., Volyniuk D., Andruleviciene V., Bezvikonnyi O., Grazulevicius J. V., Dyes and Pigments 2019, 168, 93–102; [Google Scholar]
- 11c. Chan C. Y. K., Lam J. W. Y., Zhao Z., Chen S., Lu P., Sung H. H. Y., Kwok H. S., Ma Y., Williams I. D., Tang B. Z., J. Mater. Chem. C 2014, 2, 4320–4327. [Google Scholar]
- 12.
- 12a. Yu Z., Ma B., Chen M., Wu H.-H., Liu L., Zhang J., J. Am. Chem. Soc. 2014, 136, 6904–6907; [DOI] [PubMed] [Google Scholar]
- 12b. Ma B., Wu J., Liu L., Zhang J., Chem. Commun. 2017, 53, 10164–10167; [DOI] [PubMed] [Google Scholar]
- 12c. Liu Y., Yu Z., Luo Z., Zhang J. Z., Liu L., Xia F., Zhang J., Chem. Sci. 2016, 7, 1988–1995; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12d. Xi Y., Su Y., Yu Z., Dong B., McClain E. J., Lan Y., Shi X., Angew. Chem. Int. Ed. 2014, 53, 9817–9821; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 9975–9979; [Google Scholar]
- 12e. Yu Z., Li Y., Zhang P., Liu L., Zhang J., Chem. Sci. 2019, 10, 6553–6559; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12f. Ma B., Chu Z., Huang B., Liu Z., Liu L., Zhang J., Angew. Chem. Int. Ed. 2017, 56, 2749–2753; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 2793–2797; [Google Scholar]
- 12g. López E., Borge J., López L. A., Chem. Eur. J. 2017, 23, 3091–3097; [DOI] [PubMed] [Google Scholar]
- 12h. Wang Z., Xu G., Tang S., Shao Y., Sun J., Org. Lett. 2019, 21, 8488–8491. [DOI] [PubMed] [Google Scholar]
- 13.
- 13a. Schießl J., Schulmeister J., Doppiu A., Wörner E., Rudolph M., Karch R., Hashmi A. S. K., Adv. Synth. Catal. 2018, 360, 2493–2502; [Google Scholar]
- 13b. Schießl J., Schulmeister J., Doppiu A., Wörner E., Rudolph M., Karch R., Hashmi A. S. K., Adv. Synth. Catal. 2018, 360, 3949–3959. [Google Scholar]
- 14. Singh R. K. R., Liu R.-S., Chem. Commun. 2017, 53, 4593–4596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary