Abstract
Hepatitis E virus infection can cause chronic hepatitis in immunocompromised patients with significant chance of progressive fibrosis and possibly cirrhosis. The aim of this systematic review was to summarize the efficacy and safety of the various treatment options for chronic hepatitis E. We performed a systematic literature search. The primary outcome measure was a sustained virological response (SVR). Secondary end points were rapid virological response (RVR), relapse rates, side effects and adverse events. Forty‐four articles were included with a total of 582 patients. Reduction of immunosuppressive medication induced viral clearance in 55/174 (32%) of the patients. Meta‐analysis of 395 patients showed a pooled SVR rate of 78% (95‐CI 72%–84%) after ribavirin treatment. Twenty‐five per cent of the patients obtained a RVR, whereas a relapse occurred in 18% of the patients. Anaemia during treatment led to dose reduction, use of erythropoietin and/or blood transfusion in 37% of the patients. A second treatment attempt with ribavirin led to a SVR in 39/51 (76%) of the patients. Pegylated interferon‐alpha was administered to 13 patients and SVR was obtained in 85%. Two patients (15%) suffered from acute transplant rejection during treatment with interferon. In conclusion, reduction of immunosuppressive medication and treatment with ribavirin is safe, generally well tolerated and induced viral clearance in 32% and 78% of patients, respectively. Therefore, ribavirin should be considered as first treatment step for chronic hepatitis E. Treatment with pegylated interferon‐alpha increases the risk of transplant rejection and should therefore be administered with great caution.
Keywords: systematic review, hepatitis E virus, ribavirin, immunosuppression
Abbreviations
- 95‐CI
95% confidence interval
- AML
acute myeloid leukaemia
- DNA
deoxyribonucleic acid
- HBV
hepatitis B virus
- HEV
hepatitis E virus
- HIV
human immunodeficiency virus
- MMF
mycophenolate mofetil
- Peg‐IFN
pegylated interferon‐α
- RBV
ribavirin
- RNA
ribonucleic acid
- RVR
rapid virological response
- SE
standard error
- SOT
solid organ transplant
- SVR
sustained virological response
1. INTRODUCTION
Hepatitis E virus (HEV) is one of the major causes of acute hepatitis worldwide. The virus is accountable for approximately 20 million infected individuals and up to 70,000 deaths globally every year. 1 The seroprevalence of HEV varies greatly across different regions in the world, ranging from 1% to 52%. The highest prevalence is seen in developing countries. 2 , 3 , 4 , 5 , 6 Currently, four of the eight identified genotypes of this single‐stranded RNA virus show the ability to infect humans. 7 Genotypes 1 and 2 are human pathogens that are feco‐orally transmitted and infection mainly occurs in developing countries. 8 , 9 Genotypes 3 and 4 are both zoonotic pathogens and endemic in developed countries. 9 , 10 These genotypes can be transmitted by the consumption of undercooked meat (pork, swine) or being in contact with such animals. 8 It is known that HEV infection can cause hepatic and extrahepatic symptoms, although in the majority of cases it runs an asymptomatic and self‐limiting course. 11
Hepatitis E virus infection can become chronic in patients who are not able to eliminate the virus. Chronic HEV infection is mainly seen in immunocompromised individuals receiving immunosuppressive medication after solid organ transplantation (SOT). However, chronic HEV infection has also been described in patients suffering from haematological diseases, human immunodeficiency virus (HIV) and even in immunocompetent patients. 8 , 9 , 12 , 13 Chronic hepatitis E can lead to liver fibrosis and eventually cirrhosis after relatively short periods of time. 14 , 15 Treatment that induces viral clearance is essential to prevent liver damage and related complications.
Treatment options for chronic HEV infection consist of reduction of immunosuppressive medication and antiviral agents such as pegylated interferon‐alpha (peg‐IFN) and ribavirin (RBV). It has been demonstrated that immunosuppressive medication enhances HEV replication in vitro and in vivo. 16 International guidelines recommend reduction of immunosuppressive medication as first‐line treatment for chronic HEV infection resulting in viral clearance in about one third of the patients. 11 , 17 Reduction of immunosuppressive medication has to be performed with frequent monitoring of the transplant to avoid rejection of the transplanted organ.
Many articles that report on the efficacy of antiviral treatment have been published over the last years, since chronic HEV infection is detected at an increasing rate. The aim of this systematic review and meta‐analysis was to summarize the evidence on the efficacy and safety of the various treatment options for chronic HEV infection.
2. METHODS
2.1. Literature search
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 18 The research protocol was registered in the PROSPERO database for systematic reviews (CRD42019120824). We performed a systematic literature search in PubMed, Embase, Cochrane Library and ClinicalTrials.gov from inception until November 2019. Details of the search strategy including key words are provided in Supporting Information (Table S1). The search was restricted to articles published in English or Dutch. Furthermore, the search was limited by source (Embase and Medline) and publication type (articles, reviews or articles in press) in Embase. We manually evaluated the references of the articles for relevant studies that were missed during the initial search.
2.2. Study selection
Screening of the articles was performed separately by two reviewers. We included studies that contained original patient data, described chronic HEV infection (defined as detectable HEV RNA by polymerase chain reaction in serum and/or stool for at least 3 months 17 , 19 ) and treatment with reduction of immunosuppressive medication, ribavirin or peg‐IFN. Exclusion criteria consisted of patients with acute hepatitis or other forms of hepatitis, case reports describing ribavirin treatment, review articles, articles without description of original patient data or efficacy of treatment, articles that were not written in English or not available in full‐text, in vitro or animal studies and conference abstracts, letters to the editor, editorials, opinion papers or commentary.
2.3. Extraction of data
Two independent reviewers performed data extraction using a standardized data extraction form. We retrieved the following data: Publication year, study design, baseline characteristics of the study population, dosage and duration of treatment, side effects or adverse events, adjustment of immunosuppressive drugs and the effect of the treatment given. SVR, sustained virological response, was defined as serum HEV RNA remaining negative for at least 3 months after cessation of therapy. Rapid virological response, RVR, was defined as undetectable serum HEV RNA within 1 month after start of treatment. Relapse was defined as detectable HEV RNA in serum and/or stool within 3 months after cessation of therapy. Patients in which serum HEV RNA remained positive despite start of antiviral treatment were classified as non‐responder.
We obtained additional information through contact with the authors if the results after treatment were described indistinctively. We excluded patients from the analysis in case no clarification was obtained. We evaluated the quality of the included studies with the use of a validated assessment tool for case series by Chambers et al. 20 We solved any disagreement between the reviewers by discussion. In case of persisting discrepancies throughout the process of study selection and data extraction, a third independent reviewer was consulted.
2.4. Aim of study
The aim of this systematic review was to compare the SVR rate of various treatment options for chronic HEV infection. As secondary end points we assessed the safety of treatment (side effects and interventions), RVR and relapse rates.
2.5. Statistical analysis
Descriptive statistics were provided to interpret the retrieved data. Median and range were displayed in case of non‐normally distributed data. Meta‐analysis with random effects was performed in OpenMetaAnalyst. 21 Review Manager (Review Manager (RevMan) [Computer program]. Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was used to create funnel plots and to conduct the quality assessment. A p < 0.05 was considered statistically significant.
3. RESULTS
The process of study selection is displayed in Figure 1. 18 We screened 2277 titles and abstracts after removal of duplicates and subsequently assessed 203 full‐text articles of which 44 were eligible for inclusion. We included 13 prospective studies, 24 retrospective studies, 3 case series and 4 case reports representing a total of 582 patients. The quality assessment showed that five studies (11.4%) met the criteria for good methodological quality, whereas we rated six studies (13.6%) as intermediate quality. We classified the majority of the studies (n = 33, 75%) as poor methodological quality, mainly due to their retrospective study design. The details of the quality assessment are provided in Supporting Information (Figure S2). Most studies (n = 39) described treatment of chronic HEV infection in a total of 488 SOT patients. This group consisted of patients who received a kidney transplant (n = 233, 47.7%), liver transplant (n = 106, 21.7%), heart transplant (n = 38, 7.8%), lung transplant (n = 13, 2.7%), stem cell transplant (n = 9, 1.8%), islet cell transplant (n = 2, 0.4%) or multiple organ transplant (n = 25, 5.1%). The transplanted organs were not specified in 62 patients (12.7%). The remaining patients suffered from haematological disorders (n = 26), rheumatoid arthritis (n = 2), HIV infection (n = 1), granulomatosis (n = 1), retroperitoneal fibrosis (n = 1), undefined CD4‐disturbance (n = 1) and multilocular fibrosis (n = 1). HEV genotype was reported in 69% of patients and showed almost exclusively genotype 3 infections (n = 393, 97%). Eight infections with genotype 4 and one infection with genotype 7 were described. The majority of patients (68%) were treated with RBV. Thirteen patients (2%) received peg‐IFN, whereas the remaining 174 patients (30%) were treated by reduction of immunosuppressive medication.
FIGURE 1.
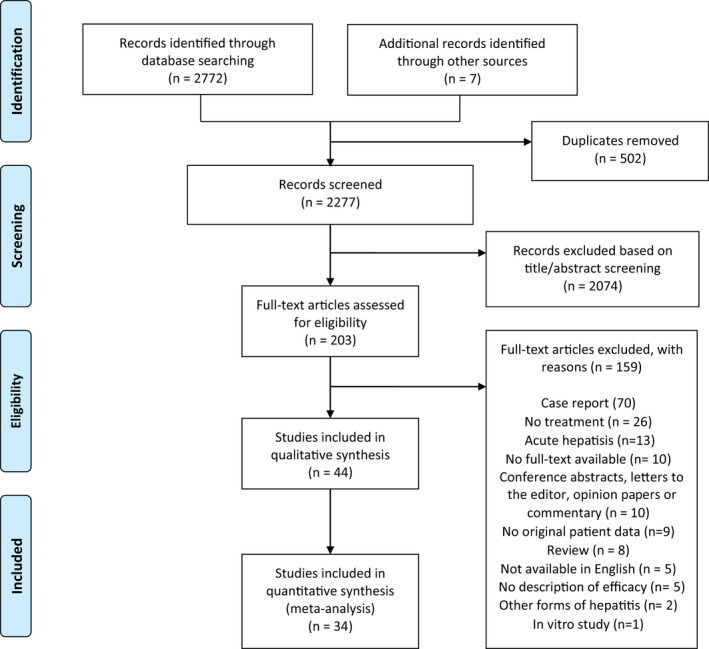
Literature search and screening process
3.1. Reduction of immunosuppressive medication
Twenty‐one studies described the effect of reduction of immunosuppressive medication, representing a total of 174 patients. 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 In most patients (67%), the reduced drug was not described. In 34 patients (20%) reduction of tacrolimus was effectuated. Mycophenolate mofetil (MMF) was reduced in 4% of the patients, whereas prednisone and cyclosporine were both reduced in 2% of the patients. Dose adjustment of azathioprine, everolimus and abatacept were all described in one individual patient. A switch in immunosuppressive medication was effectuated in two patients (1%), and a combination of agents was either reduced or stopped in nine patients (5%). The characteristics of these patients are provided in Table S3. Treatment was started after a median of 11 (2–72) months after the likely time of infection. Overall, 55 patients (32 %) achieved viral clearance (Table 2). Treatment failure, defined as serum HEV RNA remaining positive despite reduction of immunosuppressive medication, was reported in the remaining patients (n = 119, 68%). Two adverse events were observed after reduction (Table 3). Acute rejection of a kidney transplant occurred 13 months after reduction in one patients, whereas the second patient deceased due to decompensated liver cirrhosis after reduction of tacrolimus.
TABLE 2.
Pooled results from the treatment efficacy
|
RVR n (%) |
SVR n (%) |
Relapse n (%) |
Non‐response n (%) |
|
|---|---|---|---|---|
| Reduction of immunosuppressive medication (21 studies, 174 patients) | N/A | 55 (32) | N/A | 119 (68) |
| Ribavirin (34 studies, 395 patients) | 99 (25) | 301 (76) | 73 (18) | 22 (6) |
| Pegylated interferon‐α (8 studies, 13 patients) | 4 (31) | 11 (84) | 1 (8) | 1 (8) |
Abbreviations: RVR; rapid virological response (undetectable HEV RNA within one month after start of treatment); SVR, sustained virological response (undetectable HEV RNA for at least 3 months after cessation of therapy); Relapse, relapse of HEV RNA in serum and/or stool within 3 months after cessation of therapy; Non‐responder, serum HEV RNA remains positive during and after cessation of treatment.
TABLE 3.
Pooled results from the treatment safety of included studies
|
Side effects n (%) |
Intervention n (%) | Discontinuation n (%) |
Adverse events n (%) |
|
|---|---|---|---|---|
| Reduction of immunosuppressive medication (21 studies, 174 patients) | 3 (2) | 3 (2) | NA | 2 (1) |
| Ribavirin (34 studies, 395 patients) | 122 (31) | 142 (36) | 8 (2) | 4 (1) |
| Pegylated interferon‐α (8 studies, 13 patients) | 5 (38) | 5 (38) | 2 (15) | 2 (15) |
3.2. Ribavirin
Treatment effect of RBV was described in 34 studies. 22 , 23 , 25 , 26 , 27 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 The majority of the 395 patients treated with RBV was male, and almost all patients were SOT recipients. Treatment with RBV was started after a median infection duration of 8 months. In 114 patients (29%), immunosuppressive therapy was reduced before or during antiviral treatment. The characteristics of RBV therapy are provided in Table 1. Therapy was administered for a median duration of 3 months (0.3–32) in doses varying from 29 to 1200 mg/day. SVR was achieved in 301 patients (76%), whereas 22 patients (6%) were non‐responders and in 72 patients (18%) a relapse occurred. We included all studies that reported on the outcome of RBV treatment in a meta‐analysis using a random‐effects model, which showed an estimated treatment effect after RBV treatment of 78% (95‐CI 72%–84%). The results of the meta‐analysis are shown in Figure 2. We evaluated the reported outcome of RBV treatment for several underlying conditions. The results show that a SVR rate of 76% was achieved in kidney transplant recipients. Liver transplant recipients achieved a SVR rate of 91%. Lung and heart transplant recipients achieved SVR in 67% and 63% of the patients, respectively. RVR was reported in 99 patients (25%). The subsequent outcome of treatment was described in 43 of those patients: SVR was observed in 35 (81%) of these patients whereas 8 (17%) suffered from a relapse. RBV‐induced side effects were reported in 122 cases (31%). The details are provided in Table S4. The majority of patients suffered from anaemia, leading to discontinuation of treatment in 8 patients (2%). Dose reduction was effectuated in 64 patients (52%), whereas treatment with erythropoietin or blood transfusion was started in 49 patients (50%) and 4 patients (3%), respectively. A combination of interventions was applied in 17 (14%) of the subjects. Psychiatric symptoms and reluctance to RBV led to discontinuation in two patients. Four adverse events were reported during RBV treatment. Two patients deceased due to graft‐vs.‐host disease, one died from decompensated liver cirrhosis based on chronic HEV infection, whereas the other was a non‐liver related death (Table 3).
TABLE 1.
Ribavirin treatment characteristics and outcome in chronic hepatitis E patients
| Author (year) | n | Ribavirin dose, range | Duration of therapy, median (range) in months | RVR, n (%) | SVR, n (%) | Relapses, n (%) | Non‐responder, n (%) |
|---|---|---|---|---|---|---|---|
| Choi (2018) | 16 | 85.7–1000 mg/day | 3.8 (2.9) a | NR | 11 (69) | 5 (31) | |
| Cordts (2018) | 4 | 3.6–15.4 mg/kg/day | 3 (2–3) | 4 (100) | 3 (75) | 1 (25) | |
| Darstein (2018) | 4 | 400–1000 mg/day | 4 (1‐6) | 2 (50) | 4 (100) | ||
| Debing (2014) | 14 | 600–1000 mg/day | NR | NR | 13 (93) | 1 (7) | |
| Galante (2015) | 4 | 400–800 mg/day | 3 (3–6) | 1 (25) | 3 (75) | 1 (25) | |
| Gallian (2019) | 10 | NR | 5.5 (1.5–5) | NR | 8 (80) | 2 (20) | |
| Hewitt (2014) | 1 | NR | 3 | 1 (100) | |||
| Junge (2013) | 1 | 400 mg/day | 6 | 1 (100) | |||
| Kamar (2010a) | 6 | 400–800 mg/day | 3 | 5 (83) | 4 (67) | 2 (33) | |
| Kamar (2012a) | 9 | NR | 3 | 5 (42)* | 9 (100) | ||
| Kamar (2014) | 59 | 29–1200 mg/day | 3 (1–18) | 32 (54) | 46 (78) | 10 (17) | 3 (5) |
| Koning (2013) | 4 | 200–600 mg/day | 6 (3–9) | 2 (50) | 1 (25) | 1 (25) | |
| Lens (2015) | 4 | 600–800 mg/day | 3 | 3 (75) | 3 (75) | 1 (25) | |
| Lhomme (2016) | 23 | 600–800 mg/day | 3 (3–18) | NR | 10 (43) | 11 (48) | 2 (9) |
| Lhomme (2019) | 41 | 200–1200 mg/day | 3 | NR | 25 (61) | 13 (32) | 3 (7) |
| Low (2019) | 9 | 400–800 mg/day | 3 | 3 (33) | 4 (44) | 2 (22) | |
| Mallet (2018) | 3 | 10 mg/kg/day | 3 | NR | 3 (100) | ||
| Marion (2018) | 43 | 9.4 (0.5) mg/kg/day a | 3 | 19 (44) | 35 (81) | 8 (19) | |
| Marion (2019) | 48 | 9.7 (3.1) mg/kg/day a | 3 (3–15) | 17 (35) | 39 (81) | 9 (19) | |
| Nijskens (2016) | 26 | 800 mg/day | 3 (0.3–18) | 7 (27) | 25 (96) | 1 (4) | |
| Owada (2020) | 3 | NR | 3 | 3 (100) | |||
| Pischke (2012) | 4 | 200‐800 mg/day | 5 | 2 (50) | 3 (75) | 1 (25) | |
| Pischke (2013) | 7 | 600–1000 mg/day | 5 | 2 (29) | 6 (86) | 1 (14) | |
| Pischke (2014) | 1 | 200–600 mg/day | 5 | 1 (100) | |||
| Pischke (2019) | 5 | 600 mg/day | 5 (3–6) | 4 (80) | 1 (20) | ||
| Reekie (2018) | 1 | NR | NR | 1 (100) | |||
| Riezebos‐Brilman (2013a) | 5 | 800 mg/day | 4 (0.5–4) | 5(100) | |||
| Riezebos‐Brilman (2013b) | 2 | 800 mg/day | 4 | 2 (100) | |||
| Sridhar (2018a) | 3 | NR | 8 (3–22) | 2 (66) | 1 (33) | ||
| Sridhar (2018b) | 1 | 800 mg/day | 8 | 1 (100) | |||
| Sridhar (2019) | 4 | 200–400 mg/day | NR | 3 (75) | 1 (25) | ||
| Todt (2016) | 9 | 600‐1000 mg/day | 5 (4–10) | 5 (56) | 4 (44) | ||
| von Felden (2019) | 19 | 5.0–22.0 mg/kg/day | 3 (1–32) | NR | 16 (84) | 3 (16) | |
| Xhaard (2019) | 2 | NR | 4.5 (3–6) | 2 (100) | |||
| Total | 395 |
29–1200 mg/day 3.6–22.0 mg/kg/day |
3 (0.3–32) | 99/395 (25) | 301/395 (76) | 72/395 (18) | 22/395 (6) |
Abbreviations: NR, not reported; RVR, rapid virological response; SVR, sustained virological response.
Mean (SD).
FIGURE 2.
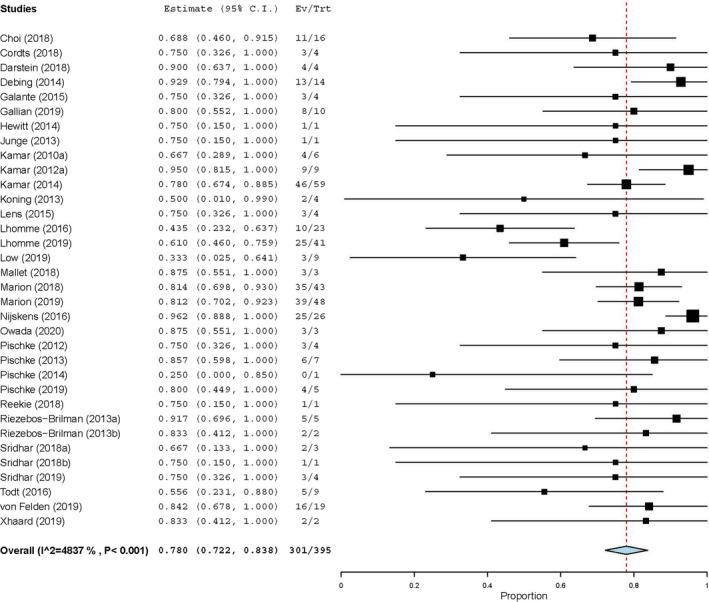
SVR rate in 34 studies that report the outcome of ribavirin treatment in patients with chronic hepatitis E. 95% CI, 95%‐confidence interval; Ev/Trt, event/treatment; HEV, hepatitis E virus; SVR, sustained virological response
In case of treatment failure, a second treatment attempt with RBV was described in 13 studies, representing 51 patients who were re‐treated for a median duration of 6 (3‐6.75) months (data not provided). Overall, SVR after re‐treatment with RBV was achieved in 39 (76%) of patients, whereas two patients (4%) suffered a relapse and HEV RNA remained detectable in 10 patients (20%). One patient deceased during the second treatment attempt due to metastatic cancer.
3.3. Pegylated interferon‐alpha
Thirteen patients were treated with Peg‐IFN. 24 , 28 , 29 , 32 , 33 , 63 , 64 , 65 Underlying diseases varied from kidney transplant patients (n = 2) and liver transplant patients (n = 5), to two patients suffering from hairy cell leukaemia and HIV, respectively. The underlying disease was not reported in three patients. Medication consisted of tacrolimus (n = 4), MMF (n = 4), steroids (n = 3), cyclosporine (n = 2) and azathioprine (n = 1). Two patients did not receive immunosuppressive medication, whereas in four patients the immunosuppressive medication regimen was not reported. In 10 patients (77%) the dose of immunosuppressive medication was reduced before or during therapy. Treatment was initiated after a median infection duration of 50 (12‐120) months. Doses ranged from 90 to 180 µg/week, given for a median duration of 3 (3–8) months. The treatment characteristics are provided in Table S5. RVR was observed in four patients (31%), whereas 11 patients (84%) achieved SVR (Table 2). Side effects were reported in five patients (Table 3) for which dose reduction was required in two patients and eventually led to treatment discontinuation in one patient. Acute transplant rejection occurred in two patients during peg‐IFN treatment which required transplantectomy in one kidney transplant patient. The other liver transplant patient was treated with steroid pulses, plasmapheresis, rituximab and increased tacrolimus dose.
3.4. Patients with HEV‐HBV or HEV‐HIV co‐infection
Four patients with chronic viral co‐infections were described. Jagjit Singh et al. described a HIV‐infected patient who was treated with peg‐IFN for six months and achieved RVR and subsequently SVR. 66 Kamar et al. described two liver transplant patients who were co‐infected with hepatitis B virus (HBV) for which they received lamivudine prophylaxis. SVR was achieved after peg‐IFN treatment in both cases. 28 Sridhar et al. reported one liver transplant patient receiving RBV alongside entecavir treatment for chronic hepatitis B. 67 HEV clearance was achieved after a treatment duration of 8 months.
3.5. Paediatric patients
In total, six paediatric transplant patients were treated with RBV. Cordts et al. reported four paediatric renal transplant patients, of which three achieved SVR after a treatment duration of 2–3 months. One patient relapsed, though eventually achieved SVR after a second treatment course of 3 months. 68 Junge et al. described a paediatric liver transplant recipient achieving SVR after a 6‐month treatment course. 69 Sridhar et al. described a 6‐year‐old kidney transplant patient who was treated with RBV at a dose of 200 mg/day. HEV RNA remained positive throughout the duration of treatment. 60 Four of these patients (67%) suffered from symptomatic anaemia that required dose reduction, blood transfusion or administration of erythropoietin.
4. DISCUSSION
The primary aim of this systematic review was to evaluate the efficacy and safety for the treatment of chronic HEV infection. Our main findings were that reduction of immunosuppressive medication, RBV treatment and peg‐IFN treatment induces viral clearance in 32%, 76% and 84% of the patients, respectively.
The efficacy of reduction of immunosuppressive medication that we reported in this systematic literature review is comparable to other studies and guidelines that reported on the efficacy of reduction. 17 , 31 , 70 . However, the efficacy of immunosuppressive dose reduction might be overestimated since only a minority of the studies report on its efficacy. As it is common clinical practice to reduce immunosuppressive medication as first‐line treatment, the patients who were treated with antiviral treatment failed to clear HEV infection after reduction of immunosuppressive medication. Therefore, the outcomes of reduction of immunosuppressive medication might be subjected to publication bias. Secondly, graft‐vs.‐host disease occurred in two patients after reduction of immunosuppressive medication, underlining the need for close surveillance of these patients.
In our review, the SVR rate after RBV treatment is higher than found in a previous systematic review by van Ton et al. 71 This difference could be explained by the increasing awareness among clinicians, which leads to earlier diagnosis and treatment of chronic HEV infection. As in chronic hepatitis C, early treatment of chronic HEV infection is likely to increase the SVR rate after antiviral treatment. 37 Secondly, the publication of consensus guidelines and improved knowledge of treatment strategies (optimal timing, dosing and duration of therapy) have likely contributed to increasing SVR rates. 17 We found that SVR rates in patients treated with RBV were higher (81%) in patients who achieved RVR. Interestingly, Marion et al. showed no significant differences in SVR rates between patients who achieved RVR compared with those who did not. 50 If RVR gives a higher chance of achieving SVR, one might argue that starting off with high doses of RBV could be beneficial. Although the guidelines do not provide clear recommendations on RBV dose, 17 in current clinical practice the initial RBV dosage is based on body weight. Subsequently, adjustment of the dosage should be performed based on serum ribavirin levels and the presence of side effects. 72 To elucidate the relationship between RVR and SVR, prospective studies that focus on viral kinetics during treatment are warranted. Although only a small amount of patients were described distinctively, liver transplant recipients show a tendency towards higher SVR rates (91%) when compared to kidney transplant recipients (76%). Treatment of lung and heart transplant recipients resulted in lower SVR rates of 67% and 63%, respectively. The difference in immunosuppressive medication regime might explain this difference, as lung and heart transplant patients are likely to receive higher doses of immunosuppressive medication to minimalize their risk of transplant rejection. However, since most studies do not report individual patient data, this review does not provide detailed information regarding the relation between the type of immunosuppressant and the outcomes of treatment. No other data have been published on this subject yet, emphasizing the need for further research in these patient groups.
In paediatric patients, treatment outcome is comparable to an adult patient population with SVR rates of 67%. In spite of the small number of paediatric patients, an important note is the high rate of anaemia that required interventions (67%) during RBV treatment.
Re‐treatment with RBV after treatment failure due to non‐response or relapse resulted in HEV clearance in 76% of the patients. The SVR rate of first and second treatment attempts were comparable. This is an interesting finding, since several studies have proposed that RBV treatment increases viral fitness. 40 , 73
Peg‐IFN is known to increase the risk for acute transplant rejection in kidney transplant patients, 74 , 75 but is thought to be relatively safe when administered to liver transplant patients. 17 Interestingly, one episode of acute rejection did occur in a liver transplant patient during treatment with peg‐IFN. However, treatment of hepatitis C virus with peg‐IFN in liver transplant patients was extensively studied and is reported to be safe with a low rejection risk. 76 , 77 Therefore, it seems feasible to reserve peg‐IFN as second treatment option in liver transplant recipients who do not respond to RBV treatment.
Sofosbuvir has been proposed as an alternative treatment method for chronic hepatitis E. Although it seems to have some efficacy in vitro, 78 only two out of seven patients described in case reports achieved a SVR. 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 Combination of peg‐IFN and RBV has been described in three patients, resulting in SVR in one patient. 87 , 88 , 89 Furthermore, a recently published prospective study confirmed the lack of effectiveness of sofosbuvir in the treatment for chronic hepatitis E. 90
The major limitation of this review lies within the quality of the included studies. The majority of the studies are retrospective patient cohorts with relatively small sample size and lack a control group. To the best of our knowledge, this is the first meta‐analysis evaluating the treatment effect of RBV in patients with chronic HEV infection. We used the random‐effects model to perform the meta‐analysis since there was significant heterogeneity among the included studies (p < 0.001). The funnel plot that we generated afterwards showed asymmetry. This result could be explained by the fact that all included studies lack a control group and therefore pose a significant risk of publication bias. This is reflected by the large number of studies that were rated as being of poor methodological quality. This underlines the need for further trials that compare several treatment options in the same patient group.
In conclusion, these systematic review and meta‐analysis show that RBV is a safe treatment that induces SVR in 76% of chronic hepatitis E patients. RBV could be used as first‐line treatment in patients with high rejection risk due to reduction of immunosuppressive medication. Supportive interventions are sufficient in most patients who suffer from RBV related side effects such as anaemia. Re‐treatment with RBV after treatment failure due to non‐response or relapse resulted in SVR rates of 76%. Reduction of immunosuppressive medication should be considered the first choice of treatment in patients with a low risk of transplant rejection and induces viral clearance in 32%. Peg‐IFN should only be used as third‐line treatment in liver transplant recipients with careful monitoring of the transplant, given the significant risk of transplant rejection.
DEFINITIONS
Non‐responder, serum HEV RNA remains positive despite start of antiviral treatment; Relapse, relapse of HEV RNA in serum and/or stool within 3 months after cessation of therapy; RVR, undetectable serum HEV RNA within one month after start of treatment; SVR, serum HEV RNA remains negative for at least 3 months after cessation of therapy; Treatment resistance, serum HEV RNA remains positive despite reduction of immunosuppressive medication; Viral clearance, serum HEV RNA becomes negative after reduction of immunosuppressive medication
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
Supporting information
Fig S2
Table S1
Table S3
Table S4
Table S5
ACKNOWLEDGEMENTS
The authors would like to acknowledge Paulien Wiersma, the librarian who kindly supported the process of designing our systematic literature search.
DATA AVAILABILITY STATEMENT
The authors declare that all data supporting the findings of this study are available within the article and its Supporting Information files.
References
- 1. Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55(4):988–97. [DOI] [PubMed] [Google Scholar]
- 2. Lapa D, Capobianchi MR, Garbuglia AR. Epidemiology of hepatitis E virus in European countries. Int J Mol Sci. 2015;16(10):25711–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hartl J, Otto B, Madden RG, et al. Hepatitis E seroprevalence in Europe: a meta‐analysis. Viruses. 2016;8(8):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ditah I, Ditah F, Devaki P, Ditah C, Kamath PS, Charlton M. Current epidemiology of hepatitis E virus infection in the United States: low seroprevalence in the National Health and Nutrition Evaluation Survey. Hepatology. 2014;60(3):815–22. [DOI] [PubMed] [Google Scholar]
- 5. Stramer SL, Moritz ED, Foster GA, et al. Hepatitis E virus: seroprevalence and frequency of viral RNA detection among US blood donors. Transfusion. 2016;56(2):481–88. [DOI] [PubMed] [Google Scholar]
- 6. Passos‐Castilho AM, de Sena A, Geraldo A, Spada C, Granato CF. High prevalence of hepatitis E virus antibodies among blood donors in Southern Brazil. J Med Virol. 2016;88(2):361–4. [DOI] [PubMed] [Google Scholar]
- 7. Purdy MA, Harrison TJ, Jameel S, et al. ICTV virus taxonomy profile: hepeviridae. J Gen Virol. 2017;98(11):2645–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dalton HR, Webb GW, Norton BC, Woolson KL. Hepatitis E virus: time to change the textbooks. Dig Dis. 2016;34(4):308–16. [DOI] [PubMed] [Google Scholar]
- 9. Debing Y, Moradpour D, Neyts J, Gouttenoire J. Update on hepatitis E virology: implications for clinical practice. J Hepatol. 2016;65(1):200–12. [DOI] [PubMed] [Google Scholar]
- 10. Wedemeyer H, Pischke S, Manns MP. Pathogenesis and treatment of hepatitis E virus infection. Gastroenterology. 2012;142(6):1388–97. [DOI] [PubMed] [Google Scholar]
- 11. McPherson S, Elsharkawy AM, Ankcorn M, et al. Summary of the British Transplantation Society UK Guidelines for Hepatitis e and Solid Organ Transplantation. Transplantation. 2018;102(1):15–20. [DOI] [PubMed] [Google Scholar]
- 12. Grewal P, Kamili S, Motamed D. Chronic hepatitis E in an immunocompetent patient: a case report. Hepatology. 2014;59(1):347–48. [DOI] [PubMed] [Google Scholar]
- 13. Loyrion E, Trouve‐Buisson T, Pouzol P, Larrat S, Decaens T, Payen JF. Hepatitis E virus infection after platelet transfusion in an immunocompetent trauma patient. Emerg Infect Dis. 2017;23(1):146–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kamar N, Mansuy JM, Cointault O, et al. Hepatitis E virus‐related cirrhosis in kidney‐ and kidney‐pancreas‐transplant recipients. Am J Transplant. 2008;8(8):1744–8. [DOI] [PubMed] [Google Scholar]
- 15. Gérolami R, Moal V, Colson P. Chronic hepatitis E with cirrhosis in a kidney‐transplant recipient. N Engl J Med. 2008;358(8):859–60. [DOI] [PubMed] [Google Scholar]
- 16. Wang Y, Zhou X, Debing Y, et al. Calcineurin inhibitors stimulate and mycophenolic acid inhibits replication of hepatitis E virus. Gastroenterology. 2014;146(7):1775–83. [DOI] [PubMed] [Google Scholar]
- 17. European Association for the Study of the Liver . EASL clinical practice guidelines on hepatitis E virus infection. J Hepatol. 2018;68(6):1256–71. [DOI] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kamar NR, Rostaing L, Legrand‐Abravanal F, Izopet J. How should hepatitis E virus infection be defined in organ‐transplant recipients? Am J Transplant. 2013;13:1935–36. [DOI] [PubMed] [Google Scholar]
- 20. Chambers D, Rodgers M, Woolacott N. Not only randomized controlled trials, but also case series should be considered in systematic reviews of rapidly developing technologies. J Clin Epidemiol. 2009;62(12):1253–60. [DOI] [PubMed] [Google Scholar]
- 21. Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end‐users: R as a computational back‐end. J Stat Softw. 2012;49:5. [Google Scholar]
- 22. Cordts SE, Schneble L, Schnitzler P, et al. Prevalence, morbidity, and therapy of hepatitis E virus infection in pediatric renal allograft recipients. Pediatr Nephrol. 2018;33(7):1215–25. [DOI] [PubMed] [Google Scholar]
- 23. Darstein F, Hauser F, Straub BK, et al. Hepatitis E virus genotype 3 is a common finding in liver‐transplanted patients undergoing liver biopsy for elevated liver enzymes with a low De Ritis ratio and suspected acute rejection: a real‐world cohort. Clin Transplant. 2018;32(11):e13411. [DOI] [PubMed] [Google Scholar]
- 24. Haagsma EB, Riezebos‐Brilman A, van den Berg AP, Porte RJ, Niesters HG. Treatment of chronic hepatitis E in liver transplant recipients with pegylated interferon alpha‐2b. Liver Transpl. 2010;16(4):474–7. [DOI] [PubMed] [Google Scholar]
- 25. Hewitt PE, Ijaz S, Brailsford SR, et al. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet. 2014;384(9956):1766–73. [DOI] [PubMed] [Google Scholar]
- 26. Junge N, Pischke S, Baumann U, et al. Results of single‐center screening for chronic hepatitis E in children after liver transplantation and report on successful treatment with ribavirin. Pediatr Transplant. 2013;17(4):343–7. [DOI] [PubMed] [Google Scholar]
- 27. Kamar N, Rostaing L, Abravanel F, et al. Ribavirin therapy inhibits viral replication on patients with chronic hepatitis E virus infection. Gastroenterology. 2010;139(5):1612–8. [DOI] [PubMed] [Google Scholar]
- 28. Kamar N, Rostaing L, Abravanel F, et al. Pegylated interferon‐α for treating chronic hepatitis E virus infection after liver transplantation. Clin Infect Dis. 2010;50(5):e30–e33. [DOI] [PubMed] [Google Scholar]
- 29. Kamar N, Abravanel F, Garrouste C, et al. Three‐month pegylated interferon‐alpha‐2a therapy for chronic hepatitis E virus infection in a haemodialysis patient. Nephrol Dial Transplant. 2010;25(8):2792–5. [DOI] [PubMed] [Google Scholar]
- 30. Kamar N, Bendall RP, Peron JM, et al. Hepatitis E virus and neurologic disorders. Emerg Infect Dis. 2011;17(2):173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kamar N, Garrouste C, Haagsma EB, et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140(5):1481–9. [DOI] [PubMed] [Google Scholar]
- 32. Kamar N, Weclawiak H, Guilbeau‐Frugier C, et al. Hepatitis E virus and the kidney in solid‐organ transplant patients. Transplantation. 2012;93(6):617–23. [DOI] [PubMed] [Google Scholar]
- 33. Kamar N, Izopet J, Rostaing L. No reactivation of hepatitis E virus after kidney retransplantation. Am J Transplant. 2012;12(2):507–8. [DOI] [PubMed] [Google Scholar]
- 34. Koning L, Pas SD, de Man RA, et al. Clinical implications of chronic hepatitis E virus infection in heart transplant recipients. J Heart Lung Transplant. 2013;32(1):78–85. [DOI] [PubMed] [Google Scholar]
- 35. Lens S, Mensa L, Gambato M, et al. HEV infection in two referral centers in Spain: epidemiology and clinical outcomes. J Clin Virol. 2015;63:76–80. [DOI] [PubMed] [Google Scholar]
- 36. Lhomme S, DebRoy S, Kamar N, et al. Plasma hepatitis E virus kinetics in solid organ transplant patients receiving ribavirin. Viruses. 2019;11(7):630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moal V, Legris T, Burtey S, et al. Infection with hepatitis E virus in kidney transplant recipients in southeastern France. J Med Virol. 2013;85(3):462–71. [DOI] [PubMed] [Google Scholar]
- 38. Pischke S, Hardtke S, Bode U, et al. Ribavirin treatment of acute and chronic hepatitis E: a single‐centre experience. Liver Int. 2013;33(5):722–6. [DOI] [PubMed] [Google Scholar]
- 39. Pischke S, Peron JM, von Wulffen M, et al. Chronic hepatitis E in rheumatology and internal medicine patients: a retrospective multicenter European cohort study. Viruses. 2019;11(2):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Todt D, Gisa A, Radonic A, et al. In vivo evidence for ribavirin‐induced mutagenesis of the hepatitis E virus genome. Gut. 2016;65(10):1733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Choi M, Hofmann J, Kohler A, et al. Prevalence and clinical correlates of chronic hepatitis E infection in German renal transplant recipients with elevated liver enzymes. Transplant Direct. 2018;4(2):e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Debing Y, Gisa A, Dallmeier K, et al. A mutation in the hepatitis E virus RNA polymerase promotes its replication and associates with ribavirin treatment failure in organ transplant recipients. Gastroenterology. 2014;147(5):1008–11. [DOI] [PubMed] [Google Scholar]
- 43. Galante A, Pischke S, Polywka S, et al. Relevance of chronic hepatitis E in liver transplant recipients: a real‐life setting. Transpl Infect Dis. 2015;17(4):617–22. [DOI] [PubMed] [Google Scholar]
- 44. Gallian P, Pouchol E, Djoudi R, et al. Transfusion‐transmitted hepatitis E virus infection in France. Transfus Med Rev. 2019;33(3):146–53. [DOI] [PubMed] [Google Scholar]
- 45. Kamar N, Izopet J, Tripon S, et al. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med. 2014;370(12):1111–20. [DOI] [PubMed] [Google Scholar]
- 46. Lhomme S, Kamar N, Nicot F, et al. Mutation in the Hepatitis E Virus Polymerase and Outcome of Ribavirin Therapy. Antimicrob Agents Chemother. 2015;60(3):1608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Low EXS, Tripon E, Lim K, et al. Risk factors for ribavirin treatment failure in Asian organ transplant recipients with chronic hepatitis E infection. World J Hepatol. 2019;11(6):553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mallet V, Sberro‐Soussan R, Roque‐Afonso AM, et al. Transmission of hepatitis E virus with plasma exchange in kidney transplant recipients: a retrospective cohort study. Transplantation. 2018;102(8):1351–57. [DOI] [PubMed] [Google Scholar]
- 49. Marion O, Abravanel F, Del Bello A, et al. Hepatitis E virus‐associated cryoglobulinemia in solid‐organ‐transplant recipients. Liver Int. 2018;38(12):2178–89. [DOI] [PubMed] [Google Scholar]
- 50. Marion O, Lhomme S, Del Bello A, et al. Monitoring hepatitis E virus fecal shedding to optimize ribavirin treatment duration in chronically infected transplant patients. J Hepatol. 2019;70(1):206–09. [DOI] [PubMed] [Google Scholar]
- 51. Nijskens CM, Pas SD, Cornelissen J, et al. Hepatitis E virus genotype 3 infection in a tertiary referral center in the Netherlands: Clinical relevance and impact on patient morbidity. J Clin Virol. 2016;74:82–7. [DOI] [PubMed] [Google Scholar]
- 52. Owada Y, Oshiro Y, Inagaki Y, et al. A nationwide survey of hepatitis E virus infection and chronic hepatitis in heart and kidney transplant recipients in Japan. Transplantation. 2020;104(2):437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pischke S, Stiefel P, Franz B, et al. Chronic hepatitis E in heart transplant recipients. Am J Transplant. 2012;12(11):3128–33. [DOI] [PubMed] [Google Scholar]
- 54. Pischke S, Greer M, Hardtke S, et al. Course and treatment of chronic hepatitis E virus infection in lung transplant recipients. Transpl Infect Dis. 2014;16(2):333–9. [DOI] [PubMed] [Google Scholar]
- 55. Reekie I, Irish D, Ijaz S, et al. Hepatitis E infection in stem cell and solid organ transplantpatients: a cross‐sectional study: the importance of HEV RNA screening in peri‐transplant period. J Clin Virol. 2018;107:1–5. [DOI] [PubMed] [Google Scholar]
- 56. Riezebos‐Brilman A, Verschuuren EA, van Son WJ, et al. The clinical course of hepatitis E virus infection in patients of a tertiary Dutch hospital over a 5‐year period. J Clin Virol. 2013;58(3):509–14. [DOI] [PubMed] [Google Scholar]
- 57. Riezebos‐Brilman A, Puchhammer‐Stockl E, van der Weide HY, et al. Chronic hepatitis E infection in lung transplant recipients. J Heart Lung Transplant. 2013;32(3):341–6. [DOI] [PubMed] [Google Scholar]
- 58. Sridhar S, Chan JFW, Yap DYH, et al. Genotype 4 hepatitis E virus is a cause of chronic hepatitis in renal transplant recipients in Hong Kong. J Viral Hepat. 2018;25(2):209–13. [DOI] [PubMed] [Google Scholar]
- 59. Sridhar S, Yip CCY, Wu S, et al. Rat hepatitis E virus as cause of persistent hepatitis after liver transplant. Emerg Infect Dis. 2018;24(12):2241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sridhar S, Cheng VCC, Wong SC, et al. Donor‐derived genotype 4 hepatitis E virus infection, Hong Kong, China, 2018. Emerg Infect Dis. 2019;25(3):425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. von Felden J, Alric L, Pischke S, et al. The burden of hepatitis E among patients with haematological malignancies: a retrospective European cohort study. J Hepatol. 2019;71(3):465–72. [DOI] [PubMed] [Google Scholar]
- 62. Xhaard A, Roque‐Afonso AM, Mallet V, et al. Hepatitis E and allogeneic hematopoietic stem cell transplantation: a French nationwide SFGM‐TC retrospective study. Viruses. 2019;11(7):622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Alric L, Bonnet D, Laurent G, Kamar N, Izopet J. Chronic hepatitis E virus infection: successful virologic response to pegylated interferon‐alpha therapy. Ann Intern Med. 2010;153(2):135–6. [DOI] [PubMed] [Google Scholar]
- 64. Jagjit Singh GK, Ijaz S, Rockwood N, et al. Chronic hepatitis E as a cause for cryptogenic cirrhosis in HIV. J Infect. 2013;66(1):103–6. [DOI] [PubMed] [Google Scholar]
- 65. Maddukuri VC, Russo MW, Ahrens WA, et al. Chronic hepatitis E with neurologic manifestations and rapid progression of liver fibrosis in a liver transplant recipient. Dig Dis Sci. 2013;58(8):2413–6. [DOI] [PubMed] [Google Scholar]
- 66. Jagjit Singh GK, Ijaz S, Rockwood N, et al. Chronic hepatitis E as a cause for cryptogenic cirrhosis in HIV. J Infect. 2013;66(1):103–106. [DOI] [PubMed] [Google Scholar]
- 67. Sridhar S, Yip CCY, Wu S, et al. Rat hepatitis E virus as cause of persistent hepatitis after liver transplant. Emerg Infect Dis. 2018;24(12):2241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cordts SE, Schneble L, Schnitzler P, et al. Prevalence, morbidity, and therapy of hepatitis E virus infection in pediatric renal allograft recipients. Pediatr Nephrol. 2018;33(7):1215–25. [DOI] [PubMed] [Google Scholar]
- 69. Junge N, Pischke S, Baumann U, et al. Results of single‐center screening for chronic hepatitis E in children after liver transplantation and report on successful treatment with ribavirin. Pediatr Transplant. 2013;17(4):343–47. [DOI] [PubMed] [Google Scholar]
- 70. Kamar N, Abravanel F, Selves J, et al. Influence of immunosuppressive therapy on the natural history of genotype 3 hepatitis‐E virus infection after organ transplantation. Transplantation. 2010;89(3):353–60. [DOI] [PubMed] [Google Scholar]
- 71. Peters Van Ton AM, Gevers TJG, Drenth JPH. Antiviral therapy in chronic hepatitis E: A systematic review. J Viral Hepat. 2015;22(12):965–73. [DOI] [PubMed] [Google Scholar]
- 72. Mulder MB, De Man RA, Kamar N, et al. Determining the therapeutic range for ribavirin in transplant recipients with chronic hepatitis E virus infection. J Viral Hepat. 2020;1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Debing Y, Ramiere C, Dallmeier K, et al. Hepatitis E virus mutations associated with ribavirin treatment failure result in altered viral fitness and ribavirin sensitivity. J Hepatol. 2016;65(3):499–508. [DOI] [PubMed] [Google Scholar]
- 74. Magnone M, Holley JL, Shapiro R. Interferon‐alpha‐induced acute renal allograft rejection. Transplantation. 1995;59:1068–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kramer PKF, Bijnen AB, Jeekel J, Weimar W. Recombinant leucocyte interferon‐a induces steroid‐resistant acute vascular rejection episodes in renal transplant recipients. Lancet. 1984;1:989. [DOI] [PubMed] [Google Scholar]
- 76. Xirouchakis E, Triantos C, Manousou P, et al. Pegylated‐interferon and ribavirin in liver transplant candidates and recipients with HCV cirrhosis: systematic review and meta‐analysis of prospective controlled studies. J Viral Hepat. 2008;15(10):699–709. [DOI] [PubMed] [Google Scholar]
- 77. Selzner N, Guindi M, Renner EL, Berenguer M. Immune‐mediated complications of the graft in interferon‐treated hepatitis C positive liver transplant recipients. J Hepatol. 2011;55(1):207–217. [DOI] [PubMed] [Google Scholar]
- 78. Dao Thi VL, Debing Y, Wu X, et al. Sofosbuvir inhibits hepatitis E virus replication in vitro and results in an additive effect when combined with ribavirin. Gastroenterology. 2016;150(1):82–85.e4. [DOI] [PubMed] [Google Scholar]
- 79. Chen J, Mehraj V, Szabo J, Routy B, Michel RP, Routy JP. Multiple remissions of extracavitary primary effusion lymphoma treated with a single cycle of liposomal doxorubicin in a patient infected with HIV. Curr Oncol. 2018;25(6):e592–e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. De Martin E, Antonini TM, Coilly A, et al. HCV and HEV recurrence after liver transplantation: one antiviral therapy for two viruses. Transpl Int. 2017;30(3):318–19. [DOI] [PubMed] [Google Scholar]
- 81. Donnelly MC, Imlach SN, Abravanel F, et al. Sofosbuvir and daclatasvir anti‐viral therapy fails to clear HEV viremia and restore reactive T cells in a HEV/HCV co‐infected liver transplant recipient. Gastroenterology. 2017;152(1):300. [DOI] [PubMed] [Google Scholar]
- 82. Drinane M, Wang XJ, Watt K. Sofosbuvir and Ribavirin eradication of refractory hepatitis E in an immunosuppressed kidney transplant recipient. Hepatology. 2019;69(5):2297–2299. [DOI] [PubMed] [Google Scholar]
- 83. Todesco E, Demeret S, Calin R, et al. Chronic hepatitis E in HIV/HBV coinfected patient: lack of power of sofosbuvir‐ribavirin. AIDS. 2017;31(9):1346–48. [DOI] [PubMed] [Google Scholar]
- 84. Todesco E, Mazzola A, Akhavan S, et al. Chronic hepatitis E in a heart transplant patient: sofosbuvir and ribavirin regimen not fully effective. AntivirvTher. 2018;23(5):463–65. [DOI] [PubMed] [Google Scholar]
- 85. van der Valk M, Zaaijer HL, Kater AP, Schinkel J. Sofosbuvir shows antiviral activity in a patient with chronic hepatitis E virus infection. J Hepatol. 2017;66(1):242–43. [DOI] [PubMed] [Google Scholar]
- 86. Van Wezel EM, De Bruijne J, Damman K, et al. Sofosbuvir add‐on to ribavirin treatment for chronic hepatitis E virus infection in solid organ transplant recipients does not result in sustained virological response. Open Forum Infect Dis. 2019;6(8):ofz346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dalton HR, Keane FE, Bendall R, Mathew J, Ijaz S. Treatment of chronic hepatitis E in a patient with HIV infection. Ann Intern Med. 2011;155(7):479–80. [DOI] [PubMed] [Google Scholar]
- 88. Mallet V, Bruneau J, Zuber J, et al. Hepatitis E virus‐induced primary cutaneous CD30(+) T cell lymphoproliferative disorder. J Hepatol. 2017;67(6):1334–39. [DOI] [PubMed] [Google Scholar]
- 89. Mazzola A, Minh MT, Charlotte F, et al. Chronic hepatitis E viral infection after liver transplantation: a regression of fibrosis after antiviral therapy. Transplantation. 2017;101(9):2083–87. [DOI] [PubMed] [Google Scholar]
- 90. Cornberg M, Pischke S, Muller T, et al. Sofosbuvir monotherapy fails to achieve HEV RNA elimination in patients with chronic hepatitis E—the HepNet SofE pilot study. J Hepatol. 2020;73(3):696–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S2
Table S1
Table S3
Table S4
Table S5
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the article and its Supporting Information files.


