FIGURE 3.
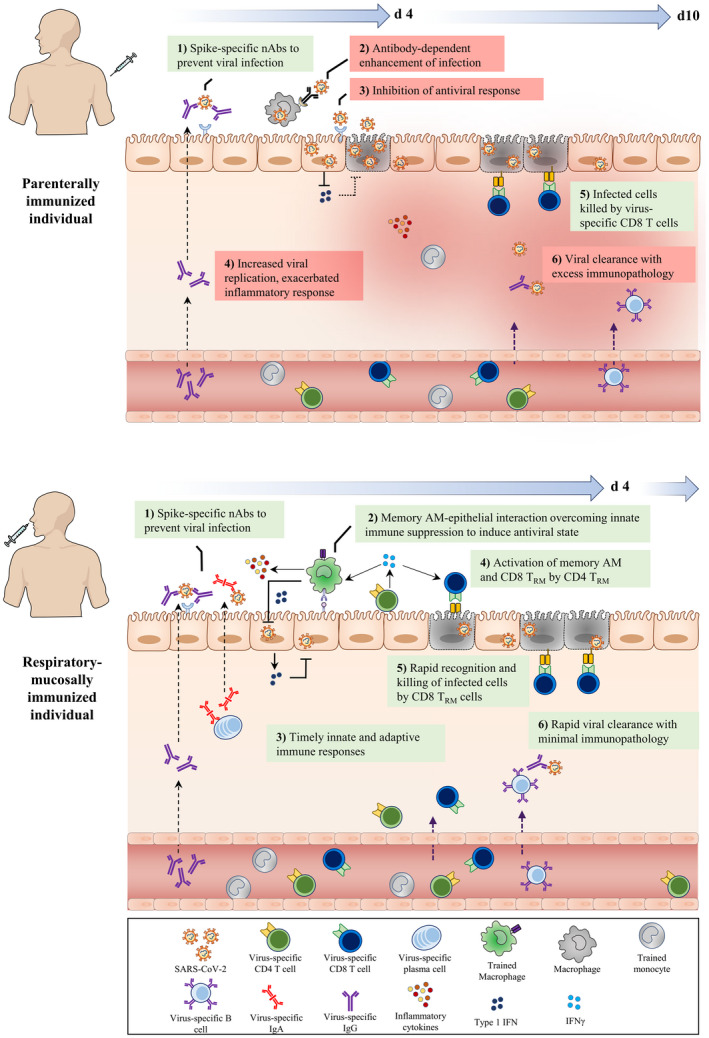
Deduced early immunological events at respiratory mucosa of parenterally or mucosally vaccinated hosts upon SARS‐CoV‐2 exposure. Following the parenteral route of immunization (top panel), circulating monocytes undergo systemic innate immune training through the priming of hematopietic monocyte progenitors in the bone marrow. Furthermore, SARS‐CoV‐2‐specific neutralizing IgG antibodies and T cells are produced and present in the circulation. However, often only the IgG antibodies are transported to the respiratory mucosal surfaces, whereas T cells and trained monocytes remain trapped within the pulmonary vasculature, resulting in an incomplete establishment of respiratory mucosal immunity. Upon SARS‐CoV‐2 infection, although the neutralizing IgG Abs (nAbs) on the surface of the respiratory mucosa bind to the incoming viruses and block its interaction with ACE2 receptor, it can be insufficient due to suboptimal levels of nAbs and weakly nAbs. However, in some hosts this mechanism may be adequate for protection. On the one hand, escaping virions may gain entry to alveolar macrophages (AM) via Ab and FcγR interaction and on the other hand, the virus infects airway epithelial cells, suppresses innate immune responses by inhibiting antiviral pathways, and delays adaptive CD8+ T cell responses. During this critical time gap (d4‐d10), poorly controlled viral replication leads to viral dissemination, dysregulated inflammatory cytokine, and inflammatory monocyte responses, resulting in excessive tissue immunopathology. In comparison, the respiratory mucosal route of immunization (bottom panel), particularly with live attenuated or viral‐vectored vaccines amenable for respiratory mucosal (RM) immunization, induces a holistic RM immunity consisting of trained alveolar macrophages (AM), mucosal IgA and IgG Abs, and lung tissue‐resident memory T (TRM) cells. RM immunization also induces a level of systemic immune protection by inducing circulating virus‐specific Abs and T cells. Upon SARS‐CoV‐2 infection, the holistic mucosal immunity overcomes the initial virus‐mediated innate immune suppression and quickly clears viral infection within the first few days (d4) via a coordinated mucosal immune response by trained (memory) AM, neutralizing mucosal Abs and TRM. nAbs (IgA/IgG) block viral entry to the epithelial cells. In dealing with the viruses that escaped from neutralization, memory AM interact with epithelial cells to overcome viral‐imposed innate immune suppression, enhancing antiviral state to inhibit viral replication. Moreover, CD4+ TRM further activate memory AM and CD8+ TRM which kill infected epithelial cells and probably infected AM. Together, such concerted immune responses lead to not only a timely control of viral infection but also prevention of excessive immunopathology and pneumonia
