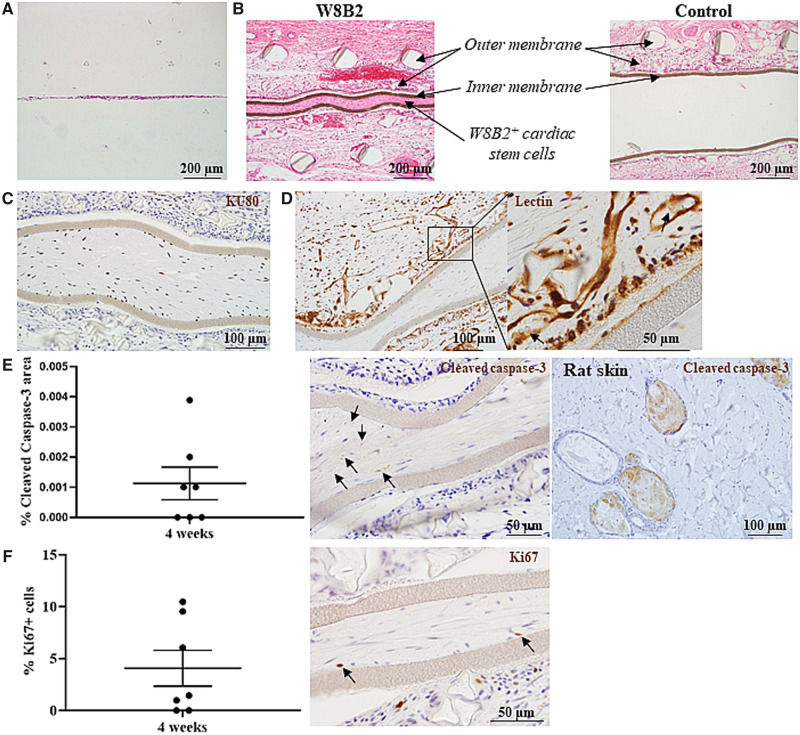
Figure 2.
Encapsulated W8B2+ CSCs in TheraCyte devices. (A, B) Eosin–haematoxylin stained Theracyte sections at 0 (A) and 4 (B) weeks post-implantation. (C–E) TheraCyte devices with encapsulated W8B2+ CSCs at 4 weeks post-implantation and stained with human-specific KU80 antibody (C), lectin (D, arrows indicate erythrocytes), cleaved caspase-3 antibody (E) and Ki67 antibody (F). (E) The percentage of cell area within the TheraCyte stained positive for cleaved caspase-3 (arrows) at Day 28 post-implantation. n = 7. (F) The percentage of Ki67 positive cells (arrows) encapsulated within the TheraCyte devices at Day 28 post-implantation. n = 7 independent experiments.