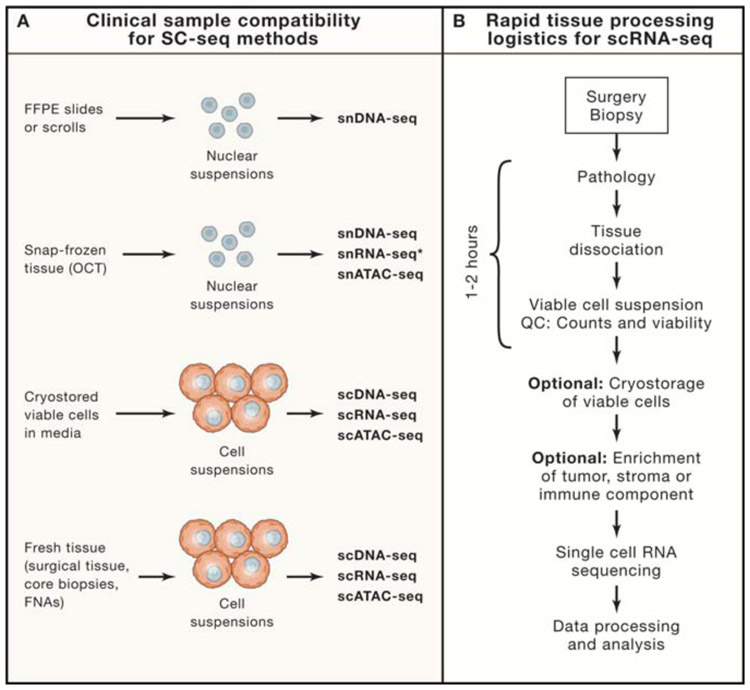
Figure 2 – Compatibility of Clinical Tissues and Processing Logistics for SCS.
(A) Clinical samples types for SCS may include FFPE tissues, snap-frozen tumors, cryostored viable cells and fresh tissues, and their compatibility with single cell and single nucleus sequencing assays is listed below. Asterisk indicates that single-nucleus RNA-seq of frozen tissues is highly dependent on the quality of the tissues. (B) Rapid tissue collection (RTC) program to procure fresh tissues collected after surgery or biopsy procedures for SCS. Optional steps include cryostorage of viable cell suspensions in freezing media and enrichment of cell types prior to running scRNA-seq assays.