Figure 4.
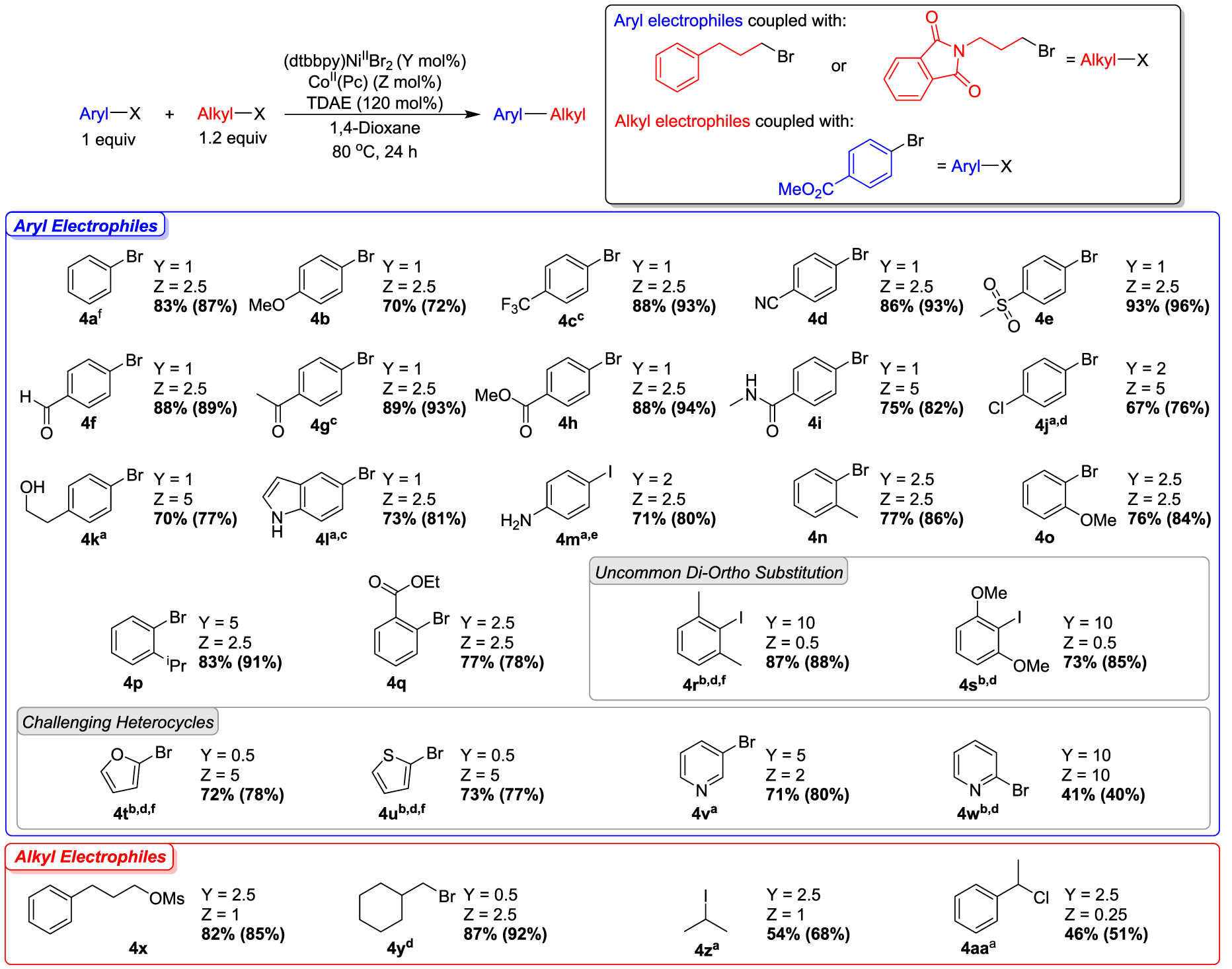
Substrate scope for dual-catalyzed cross-electrophile coupling between aryl halides and alkyl halides or pseudohalides. Values outside of parentheses are isolated yields and values inside of parentheses are NMR yields, which were determined by integration of 1H NMR spectra against a hexamethylbenzene external standard. a1.6 equiv. of alkyl substrate, 140 mol % TDAE. b2.0 equiv. of alkyl substrate, 160 mol % TDAE. c36 h. d48 h. e1-iodo-3-phenylpropane used as an alkyl substrate. fN-(3-bromopropyl)phthalimide used as an alkyl substrate instead of 1-bromo-3-phenylpropane.
