Table 3.
Cross-Electrophile Coupling of 4-tert-Butyl-Bromobenzene with 1-Bromo-3-Phenylpropane with Varying Amounts of (dtbbpy)NiIIBr2a,b
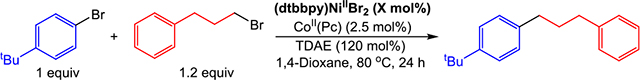 | ||||||
|---|---|---|---|---|---|---|
| entry | (dtbbpy)NiIIBr2 (X mol %) | product (%) | unreacted ArBr (%) | biphenyl (%) | unreacted AlkBr (%)c | catalytic regime |
| 1d | 0.1 | 55 | 29 | <1 | 3 | 3 |
| 2d | 0.5 | 68 | 22 | <1 | 6 | 3 |
| 3 | 1 | 84 | 6 | <1 | <1 | 2 |
| 4e | 2.5 | 66 | 4 | 9 | 10 | 1 |
| 5f | 5 | 53 | <1 | 12 | 30 | 1 |
Reaction conditions: 1-bromo-4-tertbutylbenzene (0.0625 mmol), 1-bromo-3-phenylpropane (0.075 mmol), CoII(Pc) (0.0016 mmol), and TDAE (0.075 mmol) in 1,4-dioxane (0.5 mL) at 80 °C for 24 h.
Yields are reported as the average of two trials and were determined by integration of 1H NMR spectra against a hexamethylbenzene external standard.
Yield of recovered 1-bromo-3-phenylpropane reported relative to 4-tert-butyl-bromobenzene loading.
Reaction run for 48 h.
Reaction run for 12 h.
Reaction run for 4 h.
