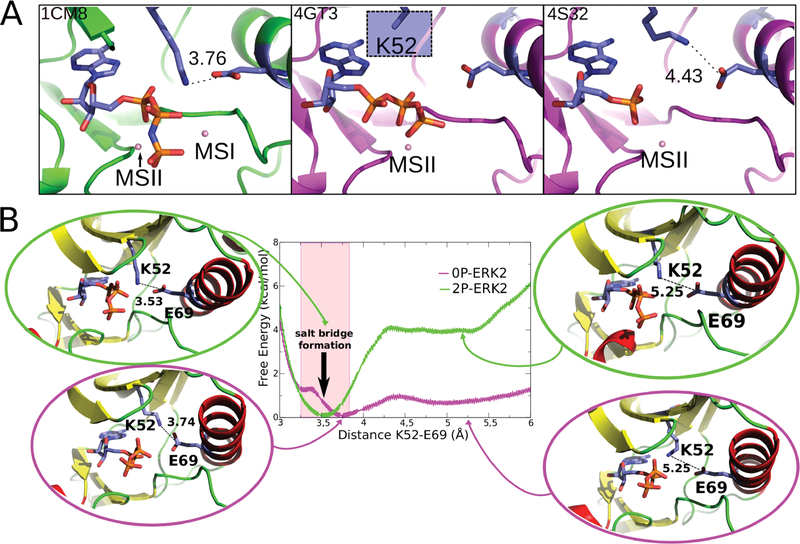
Figure 3.
Salt bridge formation between Lys52 and Glu69. (A) Representative crystallographic structures of MAPKs. 2P-P38γ (PDB ID: 1CM8; left), 0P-ERK2 (PDB ID: 4GT3, 4S32; center and right). The ligands are respectively ANP, ATP, and ANP (P-β and P-γ coordinates were not determined). The blue square (middle panel) highlights that for the Lys52 residue the side-chain is not resolved in the X-ray structure, probably due to its flexibility. (B) Free energy profile for salt-bridge formation. 0P-ERK2-ATP and 2P-ERK2-ATP are colored magenta and green, respectively. The Mg ions are not shown.