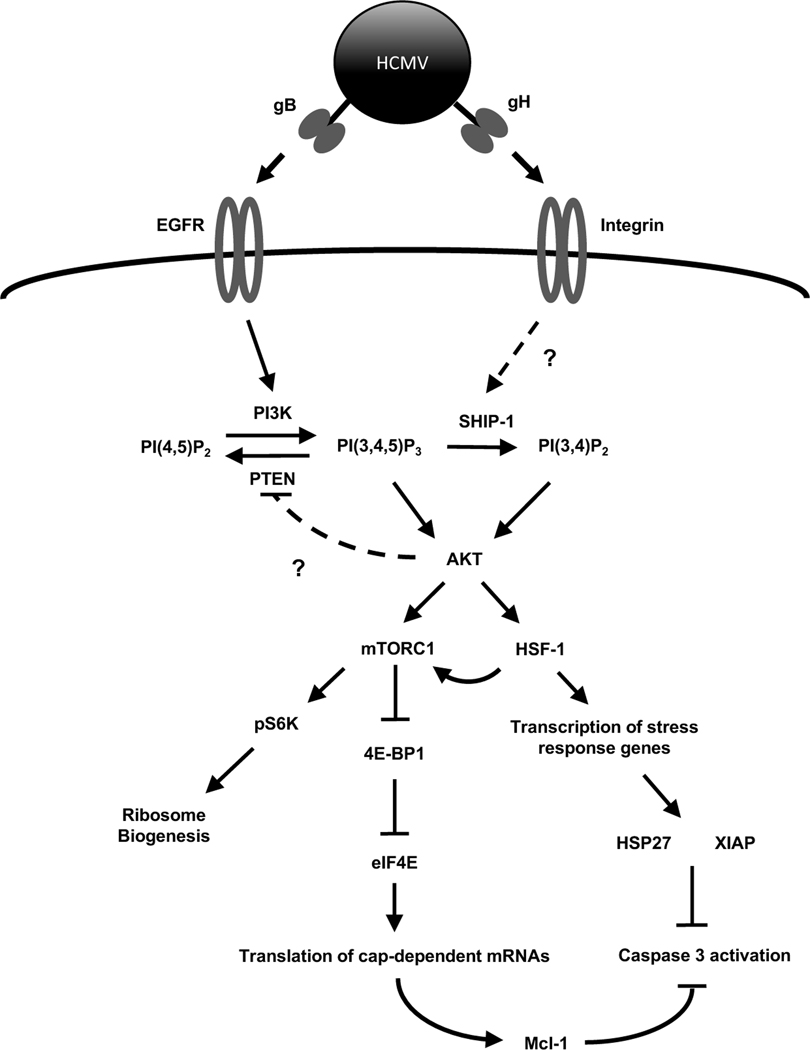
Fig. 8. Proposed model for HCMV regulation of Akt-dependent survival proteins during infection of monocytes.
Canonical Akt signaling initiated by normal myeloid growth factors induces PI3K activation through a receptor tyrosine kinase receptor. Similarly, HCMV initiation of Akt is triggered by gB binding to EGFR and the subsequent activation of PI3K. To ensure maximum Akt activity, HCMV and growth factors induce an early phosphorylation-dependent inactivation of the negative regulator PTEN by an unknown mechanism. However, unlike myeloid growth factors, HCMV concurrently stimulates a rapid upregulation of SHIP1 activity leading to the non-canonical activation of Akt. This non-traditional activation of Akt by HCMV allows for the increased translation of cap-dependent prosurvival mRNAs while also activating the stress response transcription factor HSF1. In addition to mediating an increase in stress response survival factors, HSF1 positively regulates mTOR activity resulting in the continued expression of cap-dependent antiapoptotic proteins along with stressed-induced IRES-dependent survival factors. The interplay between Akt and the stress response during HCMV infection leads to the upregulation of a unique subset of Akt-dependent antiapoptotic proteins required for the survival of infected monocytes past the critical 48-h cell-fate checkpoint.