Abstract
Background
Pulmonary metastases are a poor prognostic factor in patients with osteosarcoma; however, the clinical significance of subcentimeter lung nodules and whether they represent a tumor is not fully known. Because the clinician is faced with decisions regarding biopsy, resection, or observation of lung nodules and the potential impact they have on decisions about resection of the primary tumor, this remains an area of uncertainty in patient treatment. Surgical management of the primary tumor is tailored to prognosis, and it is unclear how aggressively patients with indeterminate pulmonary nodules (IPNs), defined as nodules smaller than 1 cm at presentation, should be treated. There is a clear need to better understand the clinical importance of these nodules.
Questions/purposes
(1) What percentage of patients with high-grade osteosarcoma and spindle cell sarcoma of bone have IPNs at diagnosis? (2) Are IPNs at diagnosis associated with worse metastasis-free and overall survival? (3) Are there any clinical or radiologic factors associated with worse overall survival in patients with IPN?
Methods
Between 2008 and 2016, 484 patients with a first presentation of osteosarcoma or spindle cell sarcoma of bone were retrospectively identified from an institutional database. Patients with the following were excluded: treatment at another institution (6%, 27 of 484), death related to complications of neoadjuvant chemotherapy (1%, 3 of 484), Grade 1 or 2 on final pathology (4%, 21 of 484) and lack of staging chest CT available for review (0.4%, 2 of 484). All patients with abnormalities on their staging chest CT underwent imaging re-review by a senior radiology consultant and were divided into three groups for comparison: no metastases (70%, 302 of 431), IPN (16%, 68 of 431), and metastases (14%, 61 of 431) at the time of diagnosis. A random subset of CT scans was reviewed by a senior radiology registrar and there was very good agreement between the two reviewers (κ = 0.88). Demographic and oncologic variables as well as treatment details and clinical course were gleaned from a longitudinally maintained institutional database. The three groups did not differ with regard to age, gender, subtype, presence of pathological fracture, tumor site, or chemotherapy-induced necrosis. They differed according to local control strategy and tumor size, with a larger proportion of patients in the metastases group presenting with larger tumor size and undergoing nonoperative treatment. There was no differential loss to follow-up among the three groups. Two percent (6 of 302) of patients with no metastases, no patients with IPN, and 2% (1 of 61) of patients with metastases were lost to follow-up at 1 year postdiagnosis but were not known to have died. Individual treatment decisions were determined as part of a multidisciplinary conference, but in general, patients without obvious metastases received (neo)adjuvant chemotherapy and surgical resection for local control. Patients in the no metastases and IPN groups did not differ in local control strategy. For patients in the IPN group, staging CT images were inspected for IPN characteristics including number, distribution, size, location, presence of mineralization, and shape. Subsequent chest CT images were examined by the same radiologist to reevaluate known nodules for interval change in size and to identify the presence of new nodules. A random subset of chest CT scans were re-reviewed by a senior radiology resident (κ = 0.62). The association of demographic and oncologic variables with metastasis-free and overall survival was first explored using the Kaplan-Meier method (log-rank test) in univariable analyses. All variables that were statistically significant (p < 0.05) in univariable analyses were entered into Cox regression multivariable analyses.
Results
Following re-review of staging chest CTs, IPNs were found in 16% (68 of 431) of patients, while an additional 14% (61 of 431) of patients had lung metastases (parenchymal nodules 10 mm or larger). After controlling for potential confounding variables like local control strategy, tumor size, and chemotherapy-induced necrosis, we found that the presence of an IPN was associated with worse overall survival and a higher incidence of metastases (hazard ratio 1.9 [95% CI 1.3 to 2.8]; p = 0.001 and HR 3.6 [95% CI 2.5 to 5.2]; p < 0.001, respectively). Two-year overall survival for patients with no metastases, IPN, or metastases was 83% [95% CI 78 to 87], 65% [95% CI 52 to 75] and 45% [95% CI 32 to 57], respectively (p = 0.001). In 74% (50 of 68) of patients with IPNs, it became apparent that they were true metastatic lesions at a median of 5.3 months. Eighty-six percent (43 of 50) of these patients had disease progression by 2 years after diagnosis. In multivariable analysis, local control strategy and tumor subtype correlated with overall survival for patients with IPNs. Patients who were treated nonoperatively and who had a secondary sarcoma had worse outcomes (HR 3.6 [95% CI 1.5 to 8.3]; p = 0.003 and HR 3.4 [95% CI 1.1 to 10.0]; p = 0.03). The presence of nodule mineralization was associated with improved overall survival in the univariable analysis (87% [95% CI 39 to 98] versus 57% [95% CI 43 to 69]; p = 0.008), however, because we could not control for other factors in a multivariable analysis, the relationship between mineralization and survival could not be determined. We were unable to detect an association between any other nodule radiologic features and survival.
Conclusion
The findings show that the presence of IPNs at diagnosis is associated with poorer survival of affected patients compared with those with normal staging chest CTs. IPNs noted at presentation in patients with high-grade osteosarcoma and spindle cell sarcoma of bone should be discussed with the patient and be considered when making treatment decisions. Further work is required to elucidate how the nodules should be managed.
Level of Evidence
Level III, prognostic study.
Introduction
The presence of lung metastases at the time of diagnosis is a poor prognostic factor in patients with primary bone sarcomas. In those with osteosarcoma, the 5-year overall survival for patients with localized disease is 60% to 80% [3, 12, 21, 26] compared with 30% to 40% for those with lung metastases [3, 12, 22]. A lung metastasis typically is defined as a parenchymal nodule with a maximal diameter of greater than 10 mm [1, 2, 9, 10, 20, 24]. Studies suggest that up to 30% of patients have subcentimeter lung nodules detected on staging chest CT images [1, 10, 15, 24]. These lesions are termed indeterminate pulmonary nodules (IPNs).
Currently, there is no consensus as to the clinical relevance of IPNs in patients with osteosarcoma. Specifically, it is unclear which IPNs will progress to lung metastases and what their association is with patient survival. Because of the small size of IPNs, biopsy is challenging, and staging is based on a radiologic evaluation. This clinical dilemma is exacerbated by the fact that subcentimeter pulmonary nodules are a common incidental finding, even in children [4, 9, 11, 18], and there are no clear radiologic findings to differentiate benign from malignant tumors [1, 4, 6, 7, 9, 10, 13, 17, 19, 23-25]. Although studies have suggested that nodules that are mineralized [4, 6], round [9], multiple in number [1, 7, 23], or are greater than 5 mm [4, 6, 17, 19, 24] have a higher chance of being malignant, others have shown no correlation between CT parameters and overall survival [10, 13, 25]. Surgical resection of the primary tumor is performed within a few months of diagnosis, and therefore, progression over time cannot be used to determine prognosis before deciding on a treatment strategy. Accurate prognosis is important for patient counselling and surgical decision-making as radical or higher-risk procedures may not be appropriate for patients whose survival is likely to be measured in months rather than years.
We therefore asked (1) What percentage of patients with high-grade osteosarcoma and spindle cell sarcoma of bone have IPNs at diagnosis? (2) Are IPNs at diagnosis associated with worse metastasis-free and overall survival? (3) Are there any clinical or radiologic factors associated with worse overall survival in patients with IPN?
Patients and Methods
Patient Selection
After obtaining institutional review board approval, we identified 484 patients with a first presentation of osteosarcoma or spindle cell sarcoma of bone diagnosed between January 1, 2008 and December 31, 2016 from a longitudinally maintained institutional database. Patients with the following were excluded: treatment at another institution (n = 27), death from chemotherapy (n = 3), Grade 1 or Grade 2 on final pathology (n = 21) and no staging chest CT performed within 2 months of diagnosis (n = 2). The remaining 431 patients underwent a retrospective review of clinical charts (Fig. 1). Demographic variables included age and sex. Oncologic variables included histologic subtype, location (axial or extremity), size (maximum diameter on MRI at presentation), and presence of extrapulmonary or skip metastases. All subtypes of high-grade osteosarcoma (such as, chondroblastic, osteoblastic, or telangiectatic) and spindle or pleomorphic sarcomas of bone were pooled as primary histologic subtypes. Tumors in radiation fields or arising from precursor lesions were grouped as secondary histologic subtypes. Treatment variables included the percentage of necrosis after chemotherapy, and management of the primary tumor (with limb salvage, amputation, or palliative). The patient’s clinical course was studied to determine metastasis-free and overall survival. The date of lung metastasis was the earliest of any of the following events: increase in the lung nodule’s size by a minimum of 25%, appearance of a new nodule on follow-up chest CT, thoracotomy with confirmatory pathologic findings, or the date the treating oncologist documented the presence of lung metastases.
Fig. 1.
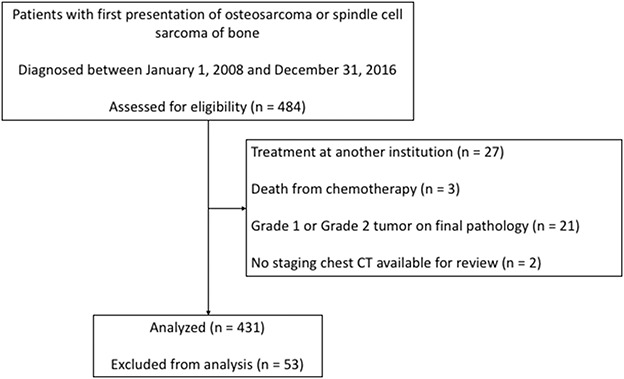
This flowchart shows the cohort included in this study.
Identification and Characterization of IPN Patients
We identified 431 patients who met our inclusion criteria. Overall survival for the entire cohort was 75% [95% CI 71 to 79] and 59% [95% CI 54 to 64] at 2 and 5 years, respectively. A multivariable analysis demonstrated improved survival for patients who received limb salvage surgery, those who had a primary sarcoma, those with a tumor size less than 8.0 cm, those with greater than 90% chemotherapy-induced necrosis, and those with localized disease on presentation (Table 1).
Table 1.
Multivariable analysis for overall survival
| Clinical variables | Patient characteristics |
Percent (n) (n = 431) |
Multivariable analysis | |||
| 2-year OS (%) | HR | 95% CI | p value | |||
| Age | < 40 years | 76 (326) | 81 | Ref | ||
| ≥ 40 years | 24 (105) | 54 | 1.3 | 0.8 to 1.8 | 0.29 | |
| Gender | Male | 58 (251) | Not included | |||
| Female | 42 (180) | Not included | ||||
| Local control | Limb salvage | 64 (276) | 83 | Ref | ||
| Amputation | 26 (113) | 74 | 1.6 | 1.1 to 2.3 | 0.008 | |
| Palliative | 10 (42) | 17 | 2.9 | 1.6 to 5.2 | < 0.001 | |
| Subtype | Primary | 92 (398) | 78 | Ref | ||
| Secondary | 8 (33) | 35 | 1.8 | 1.0 to 3.2 | 0.03 | |
| Tumor size | 0 to 7.9 cm | 24 (105) | 80 | Ref | ||
| ≥ 8.0 cm | 75 (322) | 73 | 1.6 | 1.0 to 2.3 | 0.03 | |
| Pathological fracture | No | 84 (361) | Not included | |||
| Yes | 16 (70) | Not included | ||||
| Tumor site | Extremity | 86 (371) | 80 | Ref | ||
| Axial | 14 (60) | 43 | 0.9 | 0.6 to 1.6 | 0.88 | |
| Chemotherapy-induced necrosis | < 90% | 42 (180) | 76 | Ref | ||
| ≥ 90% | 34 (146) | 94 | 0.4 | 0.3 to 0.6 | < 0.001 | |
| Stage | No metastases | 70 (302) | 83 | Ref | ||
| IPN | 16 (68) | 65 | 1.9 | 1.3 to 2.8 | 0.001 | |
| Metastases | 14 (61) | 45 | 3.0 | 2.0 to 4.4 | < 0.001 | |
OS = overall survival; HR = hazard ratio; Ref = the reference variable for multivariable analysis.
Based on an initial multidisciplinary team meeting and/or report of the local radiologist, patients were classified as follows: no metastases, metastases, and IPN at the time of diagnosis. There was no differential loss to follow-up among the three study groups; 2% (6 of 302) of no metastasis, 0% of IPN, and 2% (1 of 61) of metastasis patients were missing at one year and not known to have died (see Table 1; Supplemental Digital Content 1, http://links.lww.com/CORR/A419). All patients with lung lesions (metastases or IPN) underwent further review. Those with normal chest CTs as per the initial multidisciplinary team meeting were classified as no metastases patients and their CTs were not re-reviewed for this study. Per Rissing et al. [24], a lung metastasis was defined as a parenchymal nodule with a diameter of 10 mm or greater. Using this definition, the CT scans of all patients in the IPN category were re-reviewed in a blinded fashion by a senior consultant radiology (RB). A random subset of CT scans were reviewed by a senior radiology registrar (GA) and there was very good agreement between the two reviewers (κ = 0.88). Staging chest CT images were evaluated using an algorithm modified from guidelines published by the British Thoracic Society [5] and for lung cancer screening [16] (Fig. 2). Based on this assessment, patients with lung lesions consistent with a benign entity (for example, a pleural tag or fissural lymph node) were re-categorized into the no metastases category. A pleural tag was defined as a linear thickening of interlobular septa of lung extending to the pleura while a fissural lymph node was defined a nodule seen within or adjacent to the fissures of the lung and could be of varied shapes including oval, triangular and lentiform [8]. As per the initial multidisciplinary team meeting and/or chest CT report provided by the referring hospital, 19% (84 of 431) of patients had IPNs on presentation. After blinded re-review, 19% (16 of 84) of patients were judged to have benign pulmonary entities and not IPNs. The benign nature of these lung lesions was supported by the survival analysis; we could not detect a difference in the survival of these patients and the survival of those with normal staging studies (2-year overall survival of 72% versus 83%; HR 1.5 [95% CI 0.5 to 4.4]; p = 0.39).
Fig. 2.
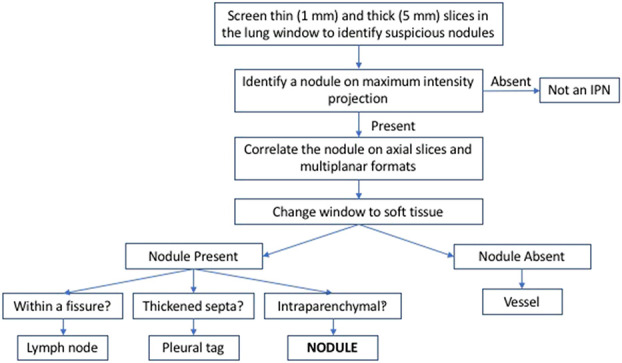
The algorithm for evaluating subcentimeter pulmonary nodules on chest CT images in our study is shown here. It was based on findings published by Callister [5] and McWilliams [16].
For patients remaining in the IPN group, the staging CT was inspected for the number of IPNs, IPN distribution (unilateral or bilateral lung fields), and largest IPN characteristics. The largest IPN characteristics were the maximum diameter (measured on lung windows), location (right lung: upper, middle, or lower lobe; left lung: upper or lower lobe), distance from the pleura (< 1 cm = peripheral; > 1 cm = central), mineralization, and shape (1 = round with clear margins; 2 = round with poorly defined margins; or 3 = lobular) (see Fig. 1A; Supplemental Digital Content 2, http://links.lww.com/CORR/A420). All subsequent chest CT images were examined to re-evaluate nodules and identify the presence of new nodules (Fig. 3). Specifically, on the restaging scan, we noted whether nodules had changed in size or mineralized after neoadjuvant chemotherapy and on all subsequent chest CTs, determined whether existing nodules increased in size and/or new nodules had appeared (see Fig. 1B; Supplemental Digital Content 2, http://links.lww.com/CORR/A420). Nodule change was assessed according to the British Thoracic Society guidelines [5] and was considered significant if the largest diameter increased or decreased by 25% or greater. Staging and re-staging chest CT images were available for 50 patients treated with neoadjuvant chemotherapy. Restaging scans were performed at a mean (range) of 2.9 months (1.6 to 5.4) after the initial CT. Pulmonary nodules diminished in 11 patients and remained stable in 18 patients. In the other 18 patients, the lung disease progressed. Again, a subset of CT images was randomly selected for review by a senior radiology trainee (GA), and there was good agreement between reviewers (κ = 0.62).
Fig. 3.
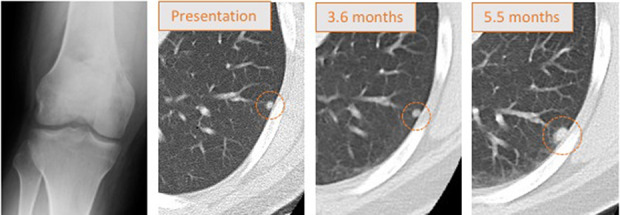
This figure displays the progression of an indeterminate pulmonary nodule. This radiograph is from a 31-year-old man with a high-grade osteoblastic osteosarcoma of the distal femur. Staging chest CT images demonstrated a single 4-mm IPN in the left lower lobe that was not mineralized. The IPN was stable on restaging chest CT and the patient had an above-the-knee amputation. Two months later, the IPN had grown and the patient died of disease 21.6 months after diagnosis.
The three groups did not differ with regard to age, gender, subtype, presence of pathological fracture, tumor site or chemotherapy-induced necrosis. They differed with regard to local control strategy and tumor size, with a larger proportion of patients in the metastases group presenting with larger tumor size and being treated nonoperatively. There were no differences in the demographic, oncologic or treatment variables studied between no metastases and IPN patients (see Table 2; Supplemental Digital Content 3, http://links.lww.com/CORR/A421).
Table 2.
Multivariable analysis for metastasis-free survival
| Clinical variables | Patient characteristics | Percent (n) (n = 370) |
2-year MFS (%) | Multivariable analysis | |||
| HR | 95% CI | p value | |||||
| Age | < 40 years | 76 (281) | Not included | ||||
| ≥ 40 years | 24 (89) | Not included | |||||
| Gender | Male | 58 (215) | Not included | ||||
| Female | 42 (155) | Not included | |||||
| Local control | Limb salvage | 68 (251) | 74 | Ref | |||
| Amputation | 26 (95) | 59 | 1.5 | 1.1 to 2.2 | 0.02 | ||
| Palliative | 6 (24) | 52 | 0.9 | 0.4 to 2.0 | 0.85 | ||
| Subtype | Primary | 92 (342) | Not included | ||||
| Secondary | 8 (28) | Not included | |||||
| Tumor size | 0 cm to 7.9 cm | 27 (99) | Not included | ||||
| ≥ 8.0 cm | 73 (271) | Not included | |||||
| Pathological fracture | No | 84 (309) | Not included | ||||
| Yes | 16 (61) | Not included | |||||
| Tumor site | Extremity | 86 (320) | Not included | ||||
| Axial | 14 (50) | Not included | |||||
| Chemotherapy-induced necrosis | < 90% | 43 (160) | 63 | Ref | |||
| ≥ 90% | 35 (129) | 83 | 0.6 | 0.4 to 0.8 | 0.005 | ||
| Stage | No metastases | 82 (302) | 77 | Ref | |||
| IPN | 18 (68) | 35 | 3.6 | 2.5 to 5.2 | < 0.001 | ||
MFS = metastasis-free survival; Ref = the reference variable for multivariable analysis.
Diagnosis and treatment were determined by a sarcoma multidisciplinary team, with chemotherapy administered per internationally agreed protocols. In general, patients without obvious metastases received (neo)adjuvant chemotherapy and surgical resection for local control. Limb salvage surgery was performed if possible with amputation reserved for unresectable tumors. Patients with clear metastatic disease had their primary tumor resected along with metastatic deposits, if possible, or as part of their palliative care plan. Patients in the no metastases and IPN groups did not differ in local control strategy, while a higher percentage of patients with metastases were treated nonoperatively (see Table 2; Supplemental Digital Content 3, http://links.lww.com/CORR/A421).
Thirty-eight percent (26 of 68) of patients with IPN at diagnosis underwent a thoracotomy at a median (range) of 11 months postdiagnosis (6 to 57).
Staging chest CTs were analyzed to identify radiologic characteristics associated with worse survival in IPN patients. Variables examined included the number of nodules (single or multiple), distribution within the lung (unilateral or bilateral lung fields), response to chemotherapy, and characteristics of the largest nodule (diameter, distance from the pleura, lobe, mineralization, and shape).
Most patients with IPN on their staging chest CT had multiple nodules (60% [41 of 68]) as well as nodules that were 5 mm or smaller (71% [48 of 68]), less than 10 mm from the pleural surface (98% [67 of 68]) and not mineralized (79% [54 of 68]). Forty-one percent (28 of 68) remained stable or shrank on neoadjuvant chemotherapy while 26% (18 of 68) of patients had nodules that grew or increased in number in this time period (see Table 3; Supplemental Digital Content 4, http://links.lww.com/CORR/A422).
Table 3.
Multivariable analysis for overall survival for patients with IPN at diagnosis
| Clinical variables | Patient characteristics | Percent (n) (n = 68) |
2-year OS (%) | Multivariable analysis | |||
| HR | 95% CI | p value | |||||
| Age | < 40 years | 76 (52) | Not included | ||||
| ≥ 40 years | 24 (16) | Not included | |||||
| Gender | Male | 59 (40) | Not included | ||||
| Female | 41 (28) | Not included | |||||
| Local control | Limb salvage | 57 (39) | 67 | Ref | |||
| Amputation | 31 (21) | 76 | 1.3 | 0.6 to 2.8 | 0.4 | ||
| Palliative | 12 (8) | 25 | 3.6 | 1.5 to 8.3 | 0.003 | ||
| Subtype | Primary | 93 (63) | 68 | Ref | |||
| Secondary | 7 (5) | 20 | 3.4 | 1.1 to 10.0 | 0.03 | ||
| Tumor size | 0-7.9 cm | 21 (14) | Not included | ||||
| ≥ 8.0 cm | 79 (54) | Not included | |||||
| Pathological fracture | No | 81 (55) | Not included | ||||
| Yes | 19 (13) | Not included | |||||
| Tumor site | Extremity | 91 (62) | Not included | ||||
| Axial | 9 (6) | Not included | |||||
| Chemotherapy-induced necrosis | < 90% | 50 (34) | Not included | ||||
| ≥ 90% | 25 (17) | Not included | |||||
| Thoracotomy | No | 60 (41) | Not included | ||||
| Yes | 38 (26) | Not included | |||||
OS = overall survival; HR = hazard ratio; Ref = the reference variable for multivariable analysis.
Statistical Analysis
The statistical analysis was performed using Prism 8 (GraphPad Software; San Diego, CA, USA) and SPSS (Version 26, IBM Corp, Armonk, NY, USA). Survival was assessed as the time between the date of diagnosis and the date of death of any cause (overall survival) or date of metastasis (according to the above-mentioned criteria). Outcomes for living patients were censored at the time of their last follow-up examination. The exploratory analysis into variables associated with overall- and metastasis-free survival was performed using the Kaplan-Meier method (log-rank test). All variables with a p < 0.05 were advanced to Cox regression multivariable analysis. Variables considered were: age at diagnosis, gender, local control strategy (limb salvage, amputation, palliative), subtype (primary, secondary), tumor size, pathological fracture, tumor site, chemotherapy-induced necrosis and stage (no metastases, IPN, metastases). For overall survival, age, local control strategy, subtype, tumor size, tumor site, chemotherapy-induced necrosis and stage advanced to multivariable analysis (Table 1), there were 25 events per variable. For metastasis-free survival, local control strategy, chemotherapy-induced necrosis and stage advanced to multivariable analysis (Table 2), there were 50 events per variable. A subgroup analysis focusing on patients with IPN was performed. For overall survival, local control strategy and subtype advanced to multivariable analysis (Table 3), there were 20 events per variable. We evaluated the presence of differences between groups using a t-test, one-way analysis of variance, and chi-square or Fisher’s exact test, as appropriate. Interrater reliability was calculated using the kappa test. Statistical significance was set at p < 0.05.
Results
Incidence of IPN in High-grade Osteosarcoma and Spindle Cell Sarcoma of Bone
Using 10 mm as the cutoff to differentiate an IPN from a lung metastasis, we found that 16% (68 of 431) of patients had an IPN on presentation. An additional 14% (61 of 431) of patients had lung metastases identified on staging chest CT images.
IPNs, Metastasis-free, and Overall Survival
Overall survival for patients with IPN was worse than for patients with no metastases on presentation (HR 1.9 [95% CI 1.3 to 2.8], p < 0.001; Fig. 4A). At 2 years, patients with IPNs had an overall survival of 65% (95% CI 52 to 75) while those with no metastases at diagnosis had an overall survival rate of 83% (95% CI 78 to 87; p < 0.001). The worst overall survival was experienced by patients with metastases, whose 2-year overall survival was 45% (HR 3.0 [95% CI 2.0 to 4.4]; p < 0.001). Similarly, patients with IPNs had worse metastasis-free survival than did patients with a normal staging chest CT (HR 3.6 [95% CI 2.5 to 5.2]; p < 0.001; Fig. 4B). At 2 years, patients with IPNs had a metastasis-free survival of 35% (95% CI 24 to 46) while those with normal staging chest CTs had a metastasis-free survival of 77% (95% CI 71 to 81; p < 0.001). Overall, metastases developed in 73% of patients with IPNs by a median (range) of 5.1 months (1.8 to 56.9), and 87% of these patients had disease progression by 24 months after diagnosis. In contrast, only 34% of patients without metastases had distant disease, and this event occurred by a median (range) of 18.5 months (0.5 to 76.0).
Fig. 4.
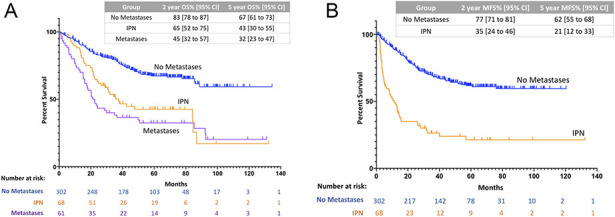
A-B Patients with IPN at diagnosis have worse overall and metastasis-free survival compared with patients with normal staging chest CTs. (A) This figure shows overall survival for patients with no metastases, IPN, or metastases at diagnosis. At 2 years, patients with IPNs had an overall survival of 65% (95% CI 52 to 75) while those with no metastases at diagnosis had an overall survival rate of 83% (95% CI 78 to 87; p < 0.001). The worst outcomes were experienced by patients with metastases, whose 2-year overall survival was 45% (95% CI 32 to 57; p < 0.001). (B) This figure shows metastasis-free survival for patients with no metastases or IPNs at diagnosis. At 2 years, patients with IPNs had a metastasis-free survival of 35% (95% CI 24 to 46) while those with normal staging chest CTs had a metastasis-free survival of 77% (95% CI 71 to 81; p < 0.001).
Factors Associated with Survival in Patients with IPN
Of the variables analyzed, only treatment of the primary tumor and tumor subtype were associated with overall survival in patients with IPNs. Among this cohort, patients with IPNs who had their primary tumor treated surgically had better survival than those treated with chemotherapy only (67% versus 25% at 2 years; HR 3.6 [95% CI 1.5 to 8.3]; p = 0.003). In addition, patients with primary tumors had improved overall survival compared with those with sarcomas secondary to radiation or predisposing lesions (68% versus 20% at 2 years; HR 3.4 [95% CI 1.1 to 10.0]; p = 0.03) (Table 3).
Presence of nodule mineralization was associated with improved overall survival in univariable analysis (87% [95% CI 39 to 98] versus 57% [95% CI 43 to 69]; p = 0.008); however, as we could not control for other factors in a multivariable analysis, the relationship between mineralization and survival could not be determined. With the numbers available, we were unable to detect an association between other CT chest findings, such as IPN size, number, shape or location, and overall survival. Similarly, IPN response to chemotherapy was not found to be related with this outcome (see Table 3; Supplemental Digital Content 4, http://links.lww.com/CORR/A422).
Discussion
Lung metastases are a poor prognostic factor in patients with high-grade osteosarcoma and spindle cell sarcoma of bone [3, 12, 22]. The size cutoff of a lung metastasis varies between 1 cm and 3 cm [1, 2, 9, 10, 20, 24]. Subcentimeter pulmonary nodules, also called IPNs, are a common incidental finding on chest CT [4, 9, 11, 18], and it is unknown how to differentiate benign from malignant nodules given that the small lesion size renders biopsy difficult. The association of IPN with metastasis-free and overall survival is unclear in this disease but critically important when determining prognosis and planning treatment. We found that patients with high-grade osteosarcoma and spindle cell sarcoma of bone who had IPN on presentation had worse metastasis-free and overall survival than patients with a normal staging chest CT. We did not identify any radiologic characteristics among patients with IPN that were associated with overall survival. We believe that the negative survival implications of IPNs on presentation should be discussed with patients and their families. This is particularly relevant when considering treatment plans that have a high risk of complications and long postoperative recovery, such as biological reconstructions. Future study is required to determine how the pulmonary nodules should be managed, specifically, the role of thoracotomy.
Limitations
This study has several limitations. First, data was drawn from a longitudinally maintained institutional database and the study is therefore subject to selection bias. As treatment details were analyzed retrospectively, we acknowledge that patients with IPN may have been treated differently than patients with normal staging chest CTs. Similarly, there may have been treatment differences within each of the three groups (no metastases, IPN, and metastases). In general, patients with both no nodules and IPN on staging chest CTs were treated with an intent-to-cure approach that included (neo)adjuvant chemotherapy and resection of the primary tumor, with either limb salvage surgery or amputation, as dictated by the tumor size and location. Patients with clear metastatic disease on presentation were treated with a palliative approach, receiving surgery for palliative purposes or chemotherapy with or without radiation. We also acknowledge the possibility of chronological variance. Although chemotherapy protocols have not changed since 2005 (the first-choice protocol since then has been high-dose methotrexate, doxorubicin, and cisplatin) and therefore should not influence results, indications for surgical approach may have varied over time due to advances in implant technology and differences in the decision-making processes of individual surgeons. We believe that patient review by an institutional multidisciplinary sarcoma team meeting reduced selection bias, and we could not detect a difference in overall treatment strategy between no metastases and IPN patients.
Second, some patients were lost to follow-up, although the numbers lost were reasonably low and did not vary between groups. This indicates that transfer bias is unlikely to have influenced our findings.
Third, patients with normal chest CTs as per the initial multidisciplinary team meeting did not have their imaging re-reviewed for this study. Consequently, there is the potential that IPN patients were underidentified if the reporting radiologists did not detect a pulmonary nodule. This would lead to an underestimation of the incidence of IPN and falsely lower metastasis-free and overall survival estimates for the no metastasis group. However, the main finding of this study would not be altered by this potential misclassification of patients between no metastasis and IPN groups.
Fourth, although we identified which IPN patients had a thoracotomy and the time interval between diagnosis and thoracotomy, due to the data collection method, we were unable to ascertain at which point in the clinical course this procedure was performed and what surgery the patient received. We recognize that thoracotomy is a known prognostic factor in this disease but also that its utility in the setting of IPN is unknown. We therefore suggest that this should be studied in future work.
Incidence of IPN in High-grade Osteosarcoma or Spindle Cell Sarcoma of Bone
We found that nearly 1 in 6 patients with high-grade osteosarcoma and spindle cell sarcoma of bone presented with IPNs at the time of diagnosis. This proportion is similar to those reported in other studies: 21% to 33% [1, 10, 15, 24]. The high incidence of IPNs found in this large series of patients adds to the existing body of evidence suggesting that subcentimeter nodules are a relevant issue in this patient population. At the same time, however, we believe that all chest CT images should be re-reviewed once the diagnosis is established in a specialized sarcoma center to ensure that patients are accurately classified.
IPNs, Metastasis-free, and Overall Survival
With the numbers available, we found that patients who presented with IPNs had worse overall survival than patients with normal staging chest CTs. The prognostic significance of IPN in patients with osteosarcoma was previously studied [10]. This group retrospectively reviewed 120 patients, 25% of whom had pulmonary nodules less than 10 mm on staging chest CT images. The authors found no correlation between IPN and poorer overall survival, but their study was smaller than ours and relied on the initial radiology report without a re-review of CT images. Another study found that patients with IPNs between 5 mm and 10 mm in diameter had worse 3-year disease-free survival than did those with no or smaller nodules [24]. This study did not address overall survival and pooled all diagnoses of bone and soft-tissue sarcomas. Similarly, we found that metastasis-free survival was worse in patients with IPNs than for the no-metastasis group. Metastases developed in 3 of 4 patients with IPNs by a median of 5.1 months, and by 2 years, most (87%) patients who would later have systemic disease had evidence of progression. Interestingly, in more than half of these patients, lung metastases occurred in a different location than did the IPN. This suggests that IPNs may represent micrometastatic disease. Our practice has been to follow patients with IPN using serial chest CTs to detect disease progression early. Although a prospective study is required to determine the natural history and timeline of IPN evolution, these preliminary findings suggest that patients should be followed radiologically using chest CT for a minimum of 2 years. Complete resection of lung metastases has been shown to have a survival advantage [14]. Given the high incidence of IPN in these patients, future work is warranted to determine whether surgical resection of these pulmonary nodules improves prognosis.
Factors Associated with Survival in Patients with IPN
Subgroup analysis of patients with IPN at presentation demonstrated that treatment with chemotherapy only and diagnosis of a secondary sarcoma were associated with worse overall survival. Although other studies have suggested that multiple IPNs [1, 7, 23], presence of mineralization [4, 6], round shape [9], and size greater than 5 mm [4, 6, 17, 19, 24] are associated with a higher chance of nodule malignancy in patients with sarcomas, we did not find a clear association between any radiologic features and overall survival. As these studies pooled a broad range of sarcoma types, used different criteria for defining an IPN, and/or included smaller numbers of patients, it is difficult to directly compare our results with the previous publications. Similarly, we did not find a correlation between IPN response to chemotherapy and patient survival. As it would be clinically useful to identify a subset of patients with IPN at diagnosis who can expect a better outcome, a multicenter study is warranted to include a sufficient number of patients with high-grade osteosarcoma to adequately address this question. Until this data is available, our results indicate that all patients with IPN on their staging chest CT should be counselled on the negative survival implications of this finding. Furthermore, the presence of IPN should be considered when deciding on a patient’s treatment plan; given their poorer overall survival, surgical procedures that involve prolonged recovery or carry a high risk of complications may not be suitable.
Conclusion
We found that IPNs were relatively common at the time of diagnosis in patients with high-grade osteosarcoma or spindle cell sarcoma of bone, and that the presence of IPNs was associated with a poorer overall survival. Most patients with IPN progress to clear metastatic disease within 2 years and as a group, have a worse metastasis-free survival than patients with normal chest CTs at diagnosis. We suggest that this finding be conveyed to patients and their families and be considered when deciding on surgical approach, as procedures with a prolonged postoperative phase may not be in their best interest. We believe that it is important for sarcoma-experienced radiologists to re-review CT images at the time of diagnosis to accurately stratify patients. Future work should investigate the best treatment strategy for managing the subcentimeter pulmonary nodules in this patient population, in particular the role of thoracotomy.
Supplementary Material
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Royal Orthopaedic Hospital, Birmingham, United Kingdom.
References
- 1.Absalon MJ, McCarville MB, Liu T, Santana VM, Daw NC, Navid F. Pulmonary nodules discovered during the initial evaluation of pediatric patients with bone and soft-tissue sarcoma. Pediatr Blood Cancer. 2008;50:1147–1153. [DOI] [PubMed] [Google Scholar]
- 2.Adibi M, Kenney PA, Thomas AZ, Borregales LD, Nogueras-González GM, Wang X, Devine CE, Karam JA, Wood CG. Prediction of pulmonary metastasis in renal cell carcinoma patients with indeterminate pulmonary nodules. Eur Urol. 2016;69:352–360. [DOI] [PubMed] [Google Scholar]
- 3.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jürgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. [DOI] [PubMed] [Google Scholar]
- 4.Brader P, Abramson SJ, Price AP, Ishill NM, Emily ZC, Moskowitz CS, La Quaglia MP, Ginsberg MS. Do characteristics of pulmonary nodules on computed tomography in children with known osteosarcoma help distinguish whether the nodules are malignant or benign? J Pediatr Surg. 2011;46:729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callister MEJ, Baldwin DR. How should pulmonary nodules be optimally investigated and managed? Lung Cancer. 2016;91:48–55. [DOI] [PubMed] [Google Scholar]
- 6.Ciccarese F, Bazzocchi A, Ciminari R, Righi A, Rocca M, Rimondi E, Picci P, Reggiani MLB, Albisinni U, Zompatori M, Vanel D. The many faces of pulmonary metastases of osteosarcoma: Retrospective study on 283 lesions submitted to surgery. Eur J Radiol. 2015;84:2679–2685. [DOI] [PubMed] [Google Scholar]
- 7.Cipriano C, Brockman L, Romancik J, Hartemayer R, Ording J, Ginder C, Krier J, Gitelis S, Kent P. The clinical significance of initial pulmonary micronodules in young sarcoma patients. J Pediatr Hematol Oncol . 2015;37:548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Hoop B, van Ginneken B, Gietema H, Prokop M. Pulmonary perifissural nodules on CT scans: Rapid growth is not a predictor of malignancy. Radiology. 2012;265:611–616. [DOI] [PubMed] [Google Scholar]
- 9.Dudeck O, Zeile M, Andreou D, Schnapauff D, Pech M, Wieners G, Ricke J, Reichardt P, Tunn PU. Computed tomographic criteria for the discrimination of subcentimeter lung nodules in patients with soft-tissue sarcomas. Clin Imag. 2011;35:174–179. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh KM, Lee LH, Beckingsale TB, Gerrand CH, Rankin KS. Indeterminate nodules in osteosarcoma: what’s the follow-up? Br J Cancer. 2018;118:634–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanamiya M, Aoki T, Yamashita Y, Kawanami S, Korogi Y. Frequency and significance of pulmonary nodules on thin-section CT in patients with extrapulmonary malignant neoplasms. Eur J Radiol. 2012;81:152–157. [DOI] [PubMed] [Google Scholar]
- 12.Janeway KA, Barkauskas DA, Krailo MD, Meyers PA, Schwartz CL, Ebb DH, Seibel NL, Grier HE, Gorlick R, Marina N. Outcome for adolescent and young adult patients with osteosarcoma. Cancer. 2012;118:4597–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kusma J, Young C, Yin H, Stanek JR, Yeager N, Aldrink JH. Pulmonary nodule size <5 mm still warrants investigation in patients with osteosarcoma and Ewing sarcoma. J Pediatr Hematol Oncol . 2017;39:184–187. [DOI] [PubMed] [Google Scholar]
- 14.Leary SES, Wozniak AW, Billups CA, Wu J, McPherson V, Neel MD, Rao BN, Daw NC. Survival of pediatric patients after relapsed osteosarcoma: The St. Jude Children's Research Hospital experience. Cancer. 2013;119:2645–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayo Z, Kennedy S, Gao Y, Miller BJ. What is the clinical importance of incidental findings on staging CT scans in patients with sarcoma? Clin Orthop Relat Res. 2018:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McWilliams A, Tammemagi MC, Mayo JR, Roberts H, Liu G, Soghrati K, Yasufuku K, Martel S, Laberge F, Gingras M, Atkar-Khattra S, Berg CD, Evans K, Finley R, Yee J, English J, Nasute P, Goffin J, Puksa S, Stewart L, Tsai S, Johnston MR, Manos D, Nicholas G, Goss GD, Seely JM, Amjadi K, Tremblay A, Burrowes P, MacEachern P, Bhatia R, Tsao M-S, Lam S. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med . 2013;369:910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murrell Z, Dickie B, Dasgupta R. Lung nodules in pediatric oncology patients: a prediction rule for when to biopsy. J Pediatr Surg. 2011;46:833–837. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura T, Matsumine A, Matsusaka M, Mizumoto K, Mori M, Yoshizaki T, Matsubara T, Asanuma K, Sudo A. Analysis of pulmonary nodules in patients with high-grade soft tissue sarcomas Rota R, ed. PLoS One. 2017;12:e0172148–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura T, Matsumine A, Niimi R, Matsubara T, Kusuzaki K, Maeda M, Tagami T, Uchida A. Management of small pulmonary nodules in patients with sarcoma. Clin Exp Metastasis. 2009;26:713–718. [DOI] [PubMed] [Google Scholar]
- 20.Nordholm-Carstensen A, Wille-Jørgensen PA, Jorgensen LN, Harling H. Indeterminate pulmonary nodules at colorectal cancer staging: a systematic review of predictive parameters for malignancy. Ann Surg Oncol. 2013;20:4022–4030. [DOI] [PubMed] [Google Scholar]
- 21.Ogura K, Fujiwara T, Yasunaga H, Matsui H, Jeon D-G, Cho WH, Hiraga H, Ishii T, Yonemoto T, Kamoda H, Ozaki T, Kozawa E, Nishida Y, Morioka H, Hiruma T, Kakunaga S, Ueda T, Tsuda Y, Kawano H, Kawai A. Development and external validation of nomograms predicting distant metastases and overall survival after neoadjuvant chemotherapy and surgery for patients with nonmetastatic osteosarcoma: A multi-institutional study. Cancer. 2015;121:3844–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picci P, Mercuri M, Ferrari S, Alberghini M, Briccoli A, Ferrari C, Pignotti E, Bacci G. Survival in high-grade osteosarcoma: improvement over 21 years at a single institution. Ann Oncol. 2010;21:1366–1373. [DOI] [PubMed] [Google Scholar]
- 23.Picci P, Vanel D, Briccoli A, Talle K, Haakenaasen U, Malaguti C, Monti C, Ferrari C, Bacci G, Saeter G, Alvegard TA. Computed tomography of pulmonary metastases from osteosarcoma: the less poor technique. A study of 51 patients with histological correlation. Ann Oncol. 2001;12:1601–1604. [DOI] [PubMed] [Google Scholar]
- 24.Rissing S, Rougraff BT, Davis K. Indeterminate pulmonary nodules in patients with sarcoma affect survival. Clin Orthop Relat Res. 2007;459:118–121. [DOI] [PubMed] [Google Scholar]
- 25.Silva CT, Amaral JG, Moineddin R, Doda W, Babyn PS. CT characteristics of lung nodules present at diagnosis of extrapulmonary malignancy in children. AJR Am J Roentgenol. 2010;194:772–778. [DOI] [PubMed] [Google Scholar]
- 26.Smeland S, Bielack SS, Whelan J, Bernstein M, Hogendoorn P, Krailo MD, Gorlick R, Janeway KA, Ingleby FC, Anninga J, Antal I, Arndt C, Brown KLB, Butterfass-Bahloul T, Calaminus G, Capra M, Dhooge C, Eriksson M, Flanagan AM, Friedel G, Gebhardt MC, Gelderblom H, Goldsby R, Grier HE, Grimer R, Hawkins DS, Hecker-Nolting S, Hall KS, Isakoff MS, Jovic G, Kühne T, Kager L, Kalle von T, Kabickova E, Lang S, Lau CC, Leavey PJ, Lessnick SL, Mascarenhas L, Mayer-Steinacker R, Meyers PA, Nagarajan R, Randall RL, Reichardt P, Renard M, Rechnitzer C, Schwartz CL, Strauss S, Teot L, Timmermann B, Sydes MR, Marina N. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer. 2019;109:36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


