ABSTRACT
Several different vaccines have been produced for human use to prevent the highly pathogenic H5N1 influenza. Some studies reported that the clinical effectiveness of influenza vaccines in older adults may be lower than in younger adults. In this study, a meta-analysis of the immunogenicity of H5N1 influenza vaccines in elderly adults was performed. Database search was conducted in EMBASE, PubMed, the Cochrane Library, Chinese VIP, Wanfang and CBM. A total of 3951 elderly adults from 10 articles were included in the meta-analysis. Compared to a single dose, two doses of H5N1 vaccines resulted in the higher seroconversion and seroprotection. For all groups treated with adjuvanted vaccines, there were significant increases (1.55- to 2.16-fold) in the seroconversion rates (SCRs) and seroprotection rates (SPRs) after two immunizations. Oil-in-water emulsion (OE)-adjuvanted 7.5 μg vaccine caused higher antibody responses than 3.75 μg of vaccine (SCR: risk ratio (RR) = 1.26 (1.19, 1.33); SPR: RR = 1.25 (1.14, 1.36)). Elderly adults exhibited slightly lower antibody responses only when given 7.5 μg of OE-adjuvanted vaccine (SCR: RR = 1.06 (1.01, 1.11)) than younger adults. After treatment with the 7.5 μg of OE-adjuvanted vaccines, the most commonly reported adverse events were injection site pain, swelling and erythema, with the incidence of 32%, 3% and 2%, respectively, and no serious adverse events were found. These data demonstrate that two doses of 7.5 µg of OE-adjuvanted H5N1 vaccine are well tolerated and induce a robust antibody response in elderly adults.
KEYWORDS: Influenza, H5N1, vaccine, immunogenicity, elderly adults
Introduction
The spread of highly pathogenic avian H5N1 and of avain A/H7N9 viruses and their virulence in humans has raised concerns about their potential to cause an influenza pandemic. The first reported case of human infection with H5N1 occurred in Hong Kong in 1997.1,2 Since then, human cases of H5N1 influenza have been detected in China, Southeast Asia, West Asia, Africa, and Europe.3,4 Epidemiological analysis of the H5N1 influenza viruses by the World Health Organization (WHO) showed that humans infected with H5N1 viruses had a mortality rate of 60%.5
Since vaccination is the main tool that can prevent influenza several different vaccines for human use have been produced against H5N1 virus and many studies were done with these vaccines. One of the first studies that assessed the immunogenicity and safety of a unadjuvanted subvirion H5N1 vaccine reported that only 58% of participants who were immunized with two doses of 90 µg each developed neutralizing antibody responses that were predicted to be associated with protection, although this vaccine was deemed to be safe.6 To enhance the immunogenicity of the H5N1 vaccine, a number of strategies have been developed in recent years. The main strategy used involves the addition of adjuvants such as aluminum (alum), MF59 and AS03 to vaccines. MF59 and AS03 are OE adjuvants using biodegradable squalene with additional emulsifying agents, which are effective adjuvants for influenza vaccine. A meta-analysis conducted by Manzoli et al7 and Guo et al8 demonstrated that, when administered at 3.75 μg or even lower doses, H5N1 vaccine supplemented with an OE adjuvant could achieve good seroprotection in healthy adults.
Older adults with poor immunity are at a higher risk of morbidity and mortality associated with influenza virus infection than younger adults.9–11 Some studies conducted in the United States showed that 60% of influenza-associated respiratory hospitalizations and over 90% of influenza-associated respiratory mortality involved adults aged ≥65 years.12,13 Vaccination against influenza is recommended annually as a key prevention strategy for elderly adults and can significantly reduce the incidence of influenza.14–17 However, vaccines are less effective in older adults because of immunosenescence,18–20 resulting in unproductive priming and recall, weakening of helper T-cells and eventual skewing of the B-cell repertoire.21 Several studies have found that influenza vaccine effectiveness in healthy adults was approximately 59%, while that in elderly adults was approximately 49%.22,23 Sobhanie et al.24 showed that the H7N9 vaccine induced significantly higher serum antibody titers in younger adults than in elderly adults. Comprehensive summaries of the available data are required to inform future public health policies regarding the use of pandemic influenza vaccines in elderly adults. The purpose of this study is to assess the immunogenicity and safety of H5N1 avian influenza vaccines in older adults in association with various adjuvants and doses and in comparison with each other.
Methods
Search strategy
Two reviewers (Ke Zhang and Xiaoxue Wu) independently searched articles in Chinese and English databases. This electronic search was conducted with combinations of the following terms: (influenza) AND (H5N1) AND (vaccines OR vaccine OR vaccination). Published studies were retrieved from the PubMed, Cochrane Library, EMBASE, MEDLINE, VIP, CBM and Wanfang databases. All retrievals were implemented by using MeSH terms and free word searches (up to January 31, 2020).
Study selection
In terms of design, we included randomized controlled trials (RCTs) and controlled before-after studies (CBAs). Articles were included in the review if they fulfilled the following criteria: (1) reported studies that investigated an H5N1 influenza vaccine, (2) included elderly adults, and (3) assessed antibody responses to an H5N1 vaccine. Studies were excluded if (1) they included only animal studies, (2) there were no elderly participants in the study, (3) they focused on research about H5N1 virus mechanisms and antiviral drugs, and (4) they did not include an H5N1 influenza vaccine.
Data extraction
Two reviewers (Ke Zhang and Xiaoxue Wu) independently extracted data from eligible studies, including data about antibody responses (SCR, SPR and geometric mean titer), vaccine-related adverse events, study design, subject ages, the number of subjects, vaccine type, the use of adjuvants, antigen dose and dosage, and the laboratory methods used for the assessment of antibody responses. Any disagreements or discrepancies were resolved by a third reviewer. The authors were contacted for clarification of data when necessary. For the results of percentage of subjects achieving a serum hemagglutination inhibition (HI) titer ≥ 1:40 displayed as images without digital data,25 images were imported into digital software (Engauge Digitizer 4.1)26 to convert the outlines into x and y coordinates. Consequently, the values of percentage were displayed and then exported into Excel files.
Assessment of the risk of bias
Study quality, risk of bias, was assessed by the Cochrane Handbook 5.1.0. The levels of risk of bias were judged as “low risk”, “high risk” and “uncertainty” in the random sequence generation, the blinding of participants and personnel, the allocation concealment, the blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias (whether the baseline is comparable).
Assessment of the immunogenicity of the H5N1 vaccine
The antibody responses to the H5N1 vaccines were assessed by antibody titers, SCR and SPR. Antibody titers were determined by HI and microneutralization (MN) assays, expressed as the reciprocal of the highest dilution at which hemagglutination was totally inhibited, or 50% neutralization of viral growth was achieved. SCR was defined as the proportion of vaccinees with either a pre-vaccination HI titer <1:10 and a post-vaccination titer ≥1:40 or a pre-vaccination HI titer ≥1:10 and a ≥ 4-fold rise in post-vaccination titer; as measured by MN assay, titer < 1:20 to ≥1:40. SPR was the percentage of participants achieving a post-vaccination serum titer of ≥1:40. The immunogenicity of the vaccines was estimated according to the criteria given by European Committee for Medicinal Products for Human Use (CHMP)27 and Center for Biologics Evaluation and Research (CBER).28 In elderly adults, if the SCR is ≥ 30% or the SPR ≥ 60%, the vaccine could be effective, and this supported the licensure of pandemic vaccines.
Statistical analysis
Comparison of sub-groups was performed using Review Manager 5.3. The RRs and the 95% confidence intervals (CIs) were calculated to estimate the differences in the SCR and SPR when one or two doses or various adjacent adjuvanted vaccine dosages were administered. Estimates of SCR and SPR were pooled using Stata12.0 software. A single-rate meta-analysis was used to assess the pooled SCR and SPR for two-dose vaccines with different adjuvants and the incidence of adverse events. Heterogeneity in the meta-analysis was assessed using the I2 statistic. The fixed-effects model (FEM) was used when I2 < 50% (p > .1), which indicated that there was no statistical heterogeneity between studies; otherwise, the random-effects model (REM) was used after excluding significant clinical heterogeneity effects if I2 ≥ 50% (p < .1). Sensitivity analysis was conducted to determine stability by deleting each study individually. Publication bias analysis was performed using Egger’s test. Cohen’s kappa statistics was performed to measure the level of agreement between reviewers on the selection of eligible studies.
Results
Literature search
A total of 7679 articles were obtained from the databases. 3116 duplicates were removed. After two selection rounds for titles and abstracts, 75 studies were found to be potentially eligible. After assessing the full texts, 10 studies were identified to meet the inclusion criteria and focus on the immunogenicity of inactivated H5N1 vaccines in elderly adults were retained for systematic review and meta-analysis,25,29–37 totaling 3951 elderly participants (Figure 1). The level of agreement between reviewers on the selection of eligible studies is very good agreement (kappa = 0.88).
Figure 1.
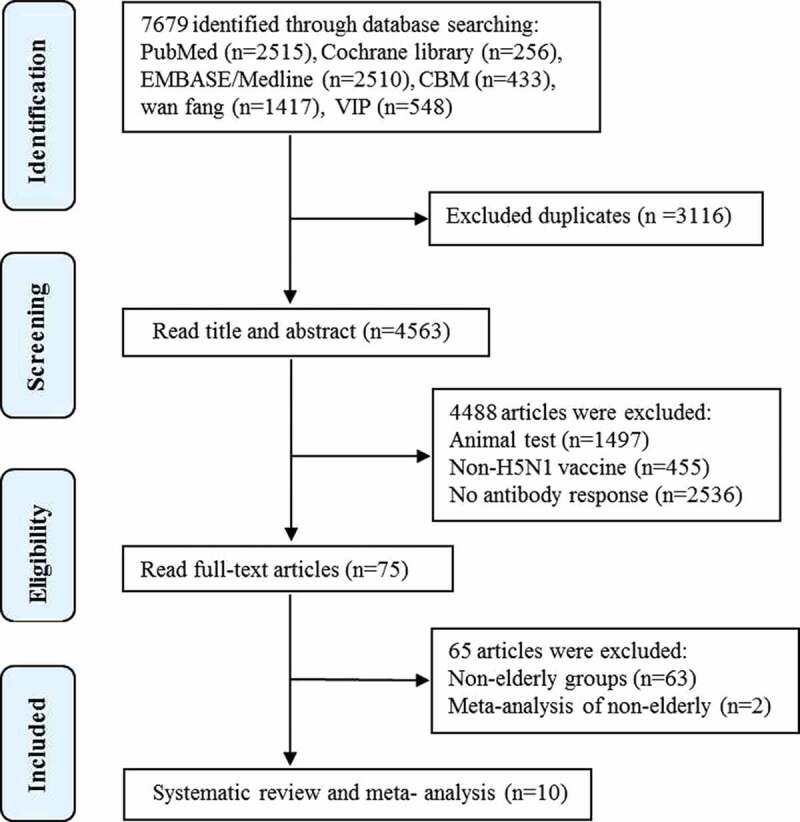
Selection process for articles used in the meta-analysis
Characteristics of the individual trials
In 729–33,35,36 of 10 included studies, adults aged 61 years or older were defined as elderly population, whereas ≥65 years and ≥60 years were chosen as age cutoff of elderly adults in two25,37 and one34 trails, respectively. The types of H5N1 vaccines used in the study included inactivated whole-virus, split-virion and subunit vaccines. Split virus vaccines contain whole inactivated viruses split with detergent, while subunit vaccines are made of purified hemagglutinin (HA) and/or neuraminidase (NA). HA and NA are the envelope glycoproteins of influenza A viruses. Most neutralizing antibodies formed in response to influenza virus are directed toward HA responsible for viral attachment to host cells. The vaccine antigen was defined as HA,25,29,32–37 or virus surface antigen including HA and NA,30,31 with doses ranging from 3.5 µg to 45 μg. The adjuvants used in these trials included OE adjuvants (MF59 and AS03) and alum. Except for one study,36 in which three groups were immunized with a single dose, all subjects were given two doses. Three studies in which participants were treated with two-dose vaccine did not have the data after the first dose. For the missing data, we have contacted the authors by e-mail. Nobody replied. The immunogenicity of the H5N1 vaccines was determined on the basis of the SCR and SPR as measured by HI or MN assays. The characteristics of the included studies are described in Table 1.
Table 1.
Characteristics of included trials
| Study | Country | Design | Age (Years) | No. | Vaccine type (Ag) | Dose (μg) + Adjuvant | Outcomes |
|---|---|---|---|---|---|---|---|
| Czajka 201230 | Poland, Turkey | RCT | 18–60 | 245 | Inactivated (HA+NA) | 3.75/7.5 + MF59 | HI, AES |
| ≥61 | 306 | ||||||
| Vesikari 201231 | Finland, Germany | RCT | 18–60 | 196 | Inactivated (HA+NA) | 7.5 + MF59 | HI |
| ≥61 | 201 | ||||||
| Bihari 201233 | Hungary | CBA | 18–60 | 193 | Inactivated (HA) | 7.5 + MF59 | HI, AES |
| ≥61 | 148 | ||||||
| Banzhoff 200932 | Italy | RCT | 18–60 | 298 | Subunit (HA) | 7.5/15 + MF59 | HI, AES |
| >60 | 155 | ||||||
| Frey 201937 | Australia, New Zealand, USA, Thailand | RCT | 18–64 | 981 | Subunit (HA) | 3.75/7.5 + MF59 | HI |
| ≥65 | 1337 | ||||||
| Heijmans 201135 | Belgian | RCT | ≥61 | 395 | Split-virion (HA) | 3.75/7.5 | HI |
| 3.75/7.5 + AS03 | HI | ||||||
| Brady 200925 | USA | RCT | ≥65 | 597 | Subunit (HA) | 3.75/7.5/15/45 | HI |
| 3.75/7.5/15/45 + Alum | HI | ||||||
| Vajo 201036 | Hungary | RCT | 18–60 | 190 | Inactivated (HA) | 3.5/6/12 + Alum | HI |
| >60 | 240 | HI | |||||
| Leroux 200929 | Belgium, UK | RCT | 18–60 | 300 | Split-virion (HA) | 7.5 | HI |
| >60 | 300 | 30 + Alum | |||||
| van der Velden 201234 | Austria, Finland, Germany | RCT | 18–59 | 270 | Inactivated (HA) | 7.5 | MN, AES |
| ≥60 | 272 |
Abbreviations: RCT, randomized controlled trial; CBA, controlled before-after study; Ag, antigen; HA, hemagglutinin; NA, neuraminidase; Alum, aluminum; HI, hemagglutination inhibition; MN, microneutralization; AES, adverse events.
Risk of bias assessment
In the ten articles included for meta-analysis, one systematic review was at low or moderate risk of bias across most domains (Figure 2). One study33 has high risk in random sequence generation and allocation concealment. Four trails29,33–35 did not perform blindness in participants and personnel, and outcome assessment. Two studies32,34 did not mention the reason for the numbers of participants who were not followed up. There is no selective reporting in all studies.
Figure 2.
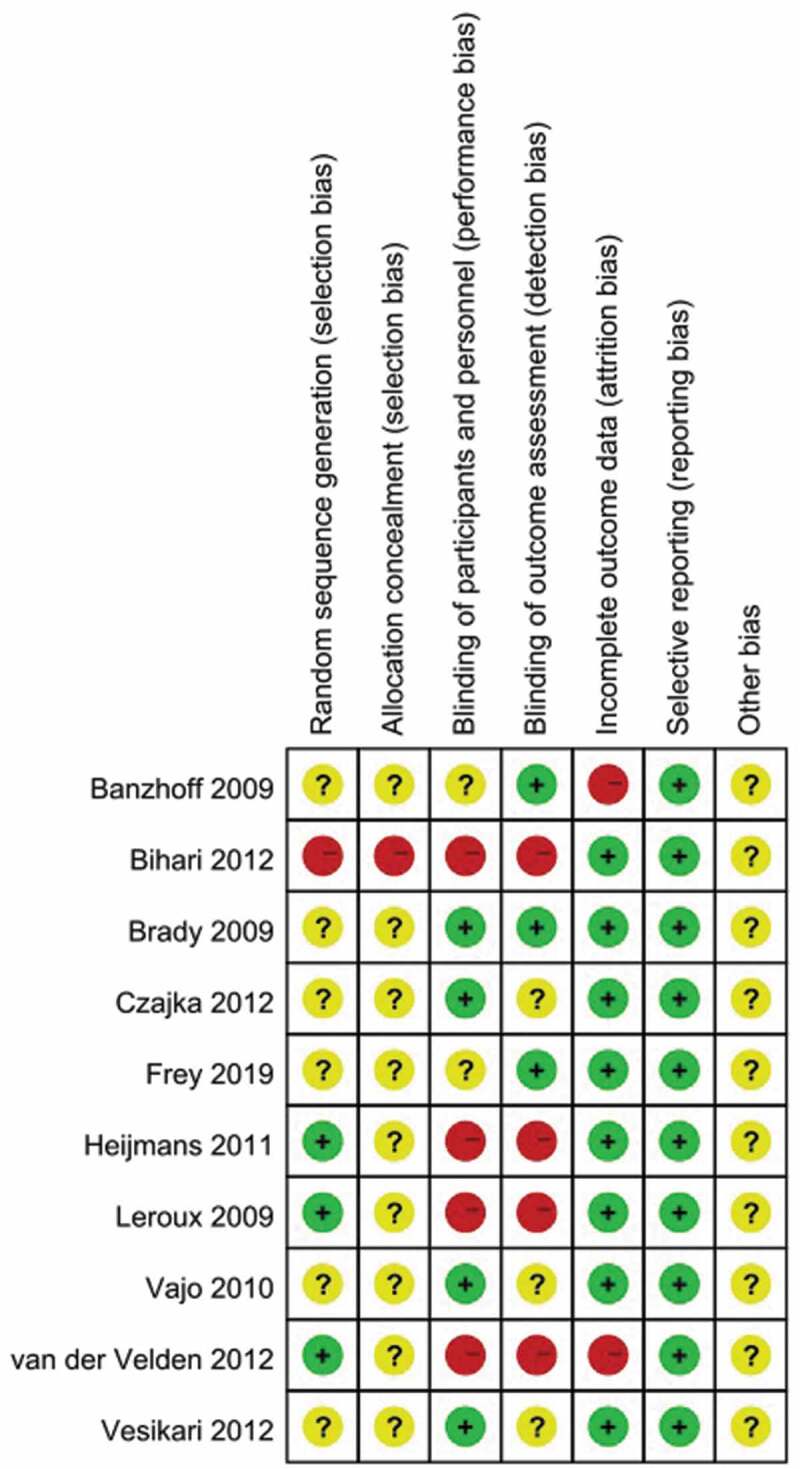
Summary of risk of bias. Plus signs, yes (low risk of bias); minus signs, no (high risk of bias); question marks, results unclear (unclear risk of bias)
Differences in the SCR and SPR resulting from a single dose and two doses
Most inactivated vaccines are poorly immunogenic in naïve subjects, and two doses of a vaccine are required to elicit a robust immune response.38,39 In this review, except for one study,36 in which three groups were immunized with a single dose of alum-adjuvanted vaccines, all subjects were given two doses. To investigate the effect of the dose on the SCR and SPR for antibodies induced by the H5N1 vaccines, the RR and the 95% CI were calculated using a random-effects model. For all adjuvant groups, there were significant increases in the SCR and SPR after two immunizations (1.55- to 2.16-fold) when compared to a single dose. For the unadjuvanted vaccines, differences were found when the antigen dose was 7.5 μg or higher (1.19- to 3.11-fold). There were no differences in the lower dose groups (3.75 μg) (Table 2). These results indicate that two doses of vaccines induce higher antibody responses than one dose.
Table 2.
Differences in the SCR and SPR resulting from a single dose and two doses
| SCR |
SPR |
|||
|---|---|---|---|---|
| Group | Studies (n) | RR (95% CI) | Studies (n) | RR (95% CI) |
| Adjuvant | ||||
| 3.75 µg | 325,30,35 | 1.87(1.32,2.67) | 230,35 | 2.16(1.05,4.42) |
| 7.5 µg | 625,30–33,35 | 1.94(1.62,2.23) | 530–33,35 | 1.97(1.63,2.39) |
| 15 µg | 225,32 | 1.55(1.17,2.0) | 132 | 1.55(1.13,2.12) |
| 45 µg | 125 | 1.60(1.03,2.48) | ||
| No Adjuvant | ||||
| 3.75 µg | 225,35 | 1.42 (0.69, 2.91) | 135 | 1.71(0.73,4.02) |
| 7.5 µg | 325,34,35 | 1.52 (1.27, 1.82) | 234,35 | 1.19(1.04,1.35) |
| 15 µg | 125 | 3.11 (1.07, 9.06) | ||
| 45 µg | 125 | 1.63 (1.05, 2.53) | ||
Abbreviations: SCR, seroconversion rate; SPR, seroprotection rate; RR, risk ratio; CI, confidence interval.
Effects of the H5N1 vaccine dose on the SCR and SPR
In this review, all trails using adjuvanted vaccines25,29–33,35-37 described higher antibody responses than unadjuvanted vaccines.25,34,35,37 Four trails25,34,35,37 assessed the immunogenicity of unadjuvanted vaccines, with different vaccine doses ranging from 3.5 to 45 μg. All unadjuvanted vaccines displayed low antibody responses in elderly adults and did not meet the CHMP or CBER criteria. To investigate the optimal doses of H5N1 vaccines in elderly adults, we analyzed the differences in the SCR and SPR resulting from subsequent doses of the two-dose of adjuvanted H5N1 vaccines. For the OE-adjuvanted vaccines, the 7.5 μg group showed higher antibody responses than the 3.75 μg group (SCR: RR = 1.26, 95% CI (1.19, 1.33); SPR: RR = 1.25, 95% CI (1.14, 1.36)). No differences were found between 7.5 μg and 15 μg OE-adjuvanted vaccines. For alum-adjuvanted vaccines, the 7.5 μg group exhibited a similar antibody response to the 3.75 μg group. Only when the antigen dose increased to 45 µg did the vaccines result in improved immunogenicity [SCR: RR = 2.48 (1.18, 5.18); SPR: RR = 2.40 (1.21, 4.57) vs 15 μg] (Figure 3).
Figure 3.
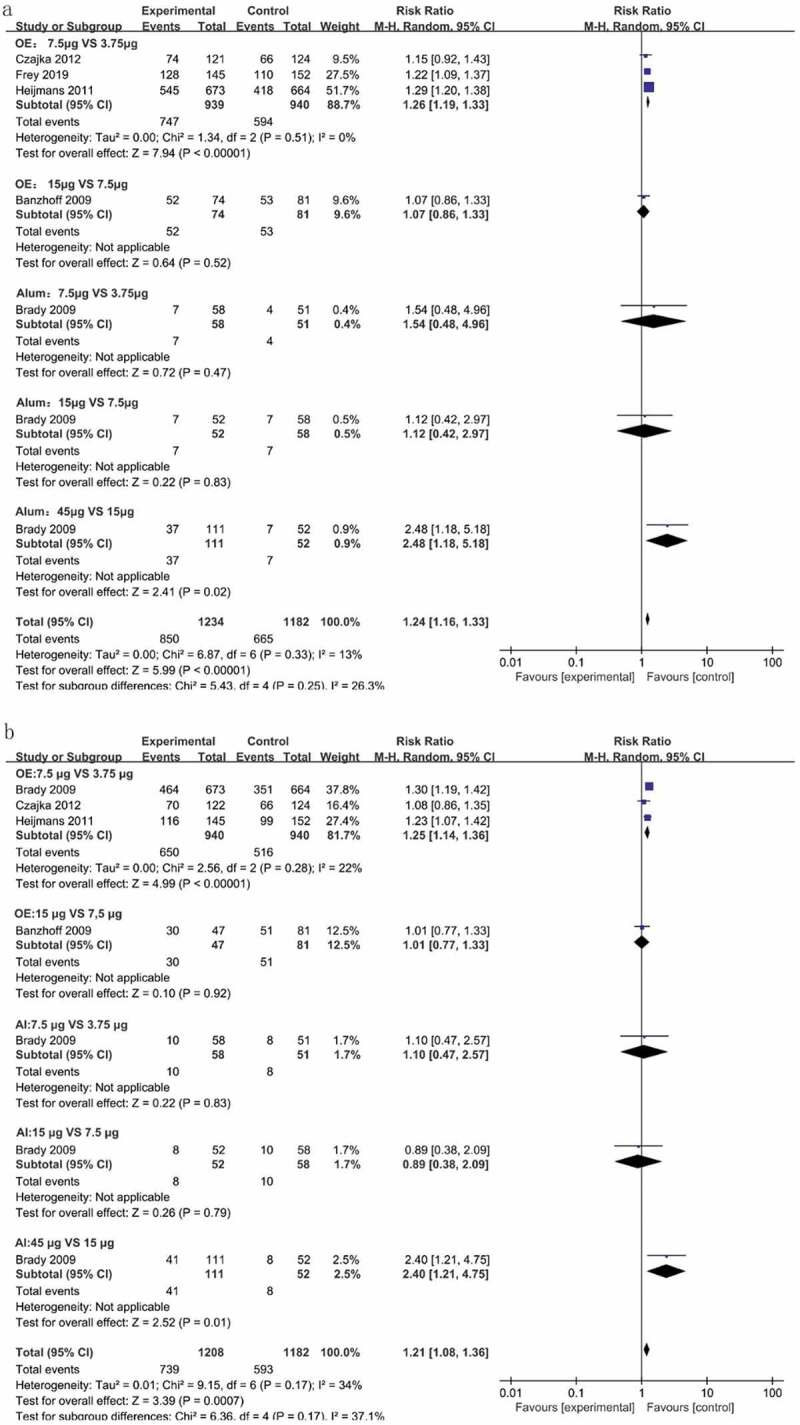
The differences in the SCR and SPR between different doses of the two-dose vaccines. The differences in the SCR (a) and SPR (b) of antibodies were analyzed between adjacent dosages of the two-dose adjuvanted H5N1 vaccines
Effects of different adjuvants on the antibody responses
Adjuvants are used to elevate the immunogenicity of influenza vaccines to produce a strong immune response in vivo.40,41 One adjuvant was used in nine of 10 included studies, either MF59, AS03 or alum, and most vaccine studies used MF59 as an adjuvant (5 of 9 studies),30–33,37 three studies used alum25,29,36 and one used AS03.35 However, in the 9 trials, only two studies set up an unadjuvanted-vaccine control (one AS03 and one alum).25,35 The AS03-adjuvanted H5N1 vaccine resulted in a higher SCR than the unadjuvanted vaccine and resulted in an RR of 3.26 (1.96, 5.42) at 3.75 μg and 3.88 (2.25, 6.72) at 7.5 μg when two doses of the H5N1 vaccines were administered.35
To investigate the differences in the immunogenicity of two-dose of H5N1 vaccines with different adjuvants, the consolidated SCR and SPR resulting from different adjuvanted vaccines were estimated using a random-effects model. As shown in Table 3, for the MF59 adjuvant, the pooled SCRs resulting from two doses of MF59-adjuvanted vaccines (3.75 μg, 7.5 μg and 15 μg) were 59%, 64% and 70%, respectively, while the pooled SPR for 3.75 μg, 7.5 μg and 15 μg MF59-adjuvanted vaccines were 53%, 62% and 65%, respectively. Only the 7.5 μg and 15 μg vaccines met the CHMP or CBER criteria, 3.75 μg not. For the AS03 adjuvant, the consolidated SCR from two-dose of AS03-adjuvanted vaccines (3.75 μg and 7.5 μg) were 72% and 88%, respectively, and the SPR were 65% and 79%, respectively. Both 3.75 μg and 7.5 μg AS03-adjuvanted vaccines met the CHMP or CBER criteria. The alum-adjuvanted vaccines showed low antibody responses, with SCR of 8%, 12% and 13% for 3.75 μg, 7.5 μg and 15 μg of vaccines, respectively, and did not meet the CHMP or CBER criteria. All unadjuvanted H5N1 vaccines did not meet the two vaccine production licensing criteria.
Table 3.
Pooled SCR and SPR of antibodies to two-dose vaccines with different adjuvants
| SCR |
SPR |
|||||
|---|---|---|---|---|---|---|
| Group | Studies (n) | Rate (95% CI) | Studies (n) | Rate (95% CI) | CHMP | CBER |
| 3.75 µg | ||||||
| No adjuvant | 225,35 | 0.13(−0.03,0.29) | 225,35 | 0.13(−0.13,0.29) | NO | NO |
| MF59 | 230,37 | 0.59(0.50,0.68) | 230,37 | 0.53(0.49,0.56) | SCR | SCR |
| AS03 | 135 | 0.72(0.65,0.79) | 135 | 0.65(0.58,0.73) | SCR/SPR | SCR/SPR |
| Alum | 125 | 0.08(0.00,0.15) | 125 | 0.16(0.06,0.26) | NO | NO |
| 7.5 µg | ||||||
| No adjuvant | 425,29,34,35 | 0.33(0.13,0.53) | 425,29,34,35 | 0.42(0.20,0.64) | SCR | SCR |
| MF59 | 530–33,37 | 0.64(0.50,0.78) | 530–33,37 | 0.62(0.56,0.68) | SCR/SPR | SCR/SPR |
| AS03 | 135 | 0.88(0.83.0.94) | 135 | 0.79(0.73,0.86) | SCR/SPR | SCR/SPR |
| Alum | 125 | 0.12(0.04,0.20) | 125 | 0.19(0.09,0.29) | NO | NO |
| 15 µg | ||||||
| No adjuvant | 125 | 0.21(0.11,0.32) | 125 | 0.29(0.17,0.40) | NO | NO |
| MF59 | 132 | 0.70(0.60,0.81) | 132 | 0.65(0.54,0.76) | SCR/SPR | SCR/SPR |
| Alum | 125 | 0.13(0.04,0.32) | 125 | 0.15(0.06,0.25) | NO | NO |
Abbreviations: Alum, aluminum; SCR, seroconversion rate; SPR, seroprotection rate; CI, confidence interval; CHMP, European Committee for Medicinal Products for Human Use; CBER, Center for Biologics Evaluation and Research.
The differences in the SCR and SPR between elderly and younger adults
Some studies had reported that the clinical effectiveness of seasonal influenza vaccines in older adults is lower than that in younger adults.18–20,42 To determine whether there is a lower immune response after H5N1 vaccination in elderly adults as compared with younger adults, we analyzed the RR of the SCR and SPR resulting from two doses of OE-adjuvanted H5N1 vaccines in 1913 younger adultsand 2147 elderly adults.30–33,37 As shown in Figure 4, elderly adults exhibited slightly lower antibody responses to 7.5 μg of inactivated H5N1vaccines than younger adults (SCR: RR = 1.06 (1.01, 1.11)). In the 3.5 μg and 15 μg HA vaccine groups, no difference in the SCRs was found between elderly and younger adults, although one study30 showed that elderly adults exhibited a lower antibody response to 3.5 μg of the H5N1 vaccine than younger adults.
Figure 4.
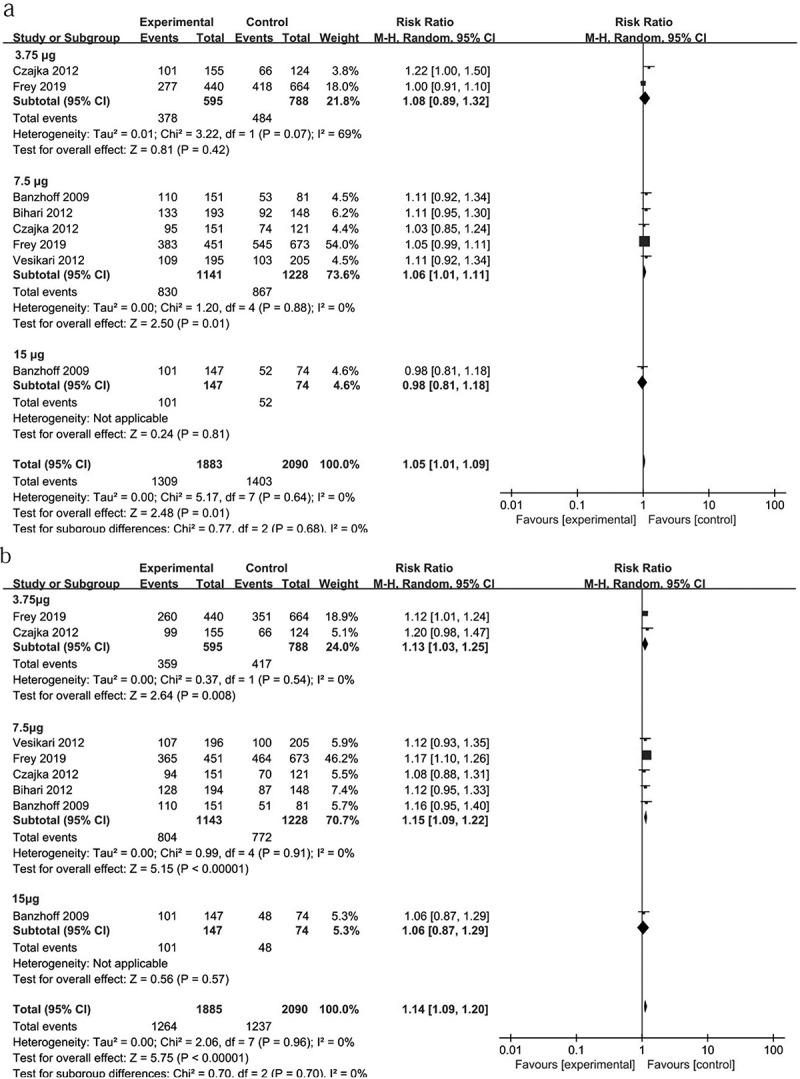
Differences in the SCR and SPR between younger and elderly adults. The differences in SCR (a) and SPR (b) for antibody responses to two doses of OE-adjuvanted H5N1 vaccines were analyzed between younger and elderly adults
Safety assessment
The vaccine-related adverse events were mild to moderate and included injection site pain, swelling, erythema, fever, headache and myalgia. We assessed the incidence of local and systemic adverse events resulting from OE-adjuvanted vaccines. The incidence of pain in participants treated with the 7.5 μg of H5N1 vaccines, that was the optimal dose for adults based on the immunogenicity and safety of vaccines,43–45 was 32% (95% CI (0, 0.63)), that of swelling was 2% (95% CI (−0.39, 0.44)) and that of erythema was 3% (95% CI (−0.05, 0.10)). The incidence of systemic adverse events, including fever, headache and myalgia, was ≤ 11%. All of the H5N1 influenza vaccines that were used in the review were well-tolerated without serious adverse events.
Sensitivity analysis
Sensitivity analysis was performed to check for instability and changes in the significance of the estimate of the effect. After each study was excluded from the meta-analysis, there was no significant shift or change in the level of significance. The studies by Vajo et al36 caused a slight shift in the SCR and significance for the 3.5–3.75 µg doses of the alum-adjuvanted vaccines, but this shift was not considered to be large due to the lower limit of the CI (−9.5, 48.9).
Publication bias
Due to the limited body of literature, funnel plots cannot be used for an adequate evaluation of the publication bias. Egger’s test was used to evaluate the publication bias in regard to antibody responses to H5N1 vaccines. The p values were greater than 0.05 in Egger’s test at adjuvanted vaccines in which most of studies were included, suggesting the absence of significant publication bias in the overall meta-analysis.
Discussion
In this study, 10 trials were found to be eligible for a systematic review and meta-analysis of antibody responses to inactivated H5N1 vaccines in elderly adults. Inactivated virus vaccines, including whole virus, split virion and subunit vaccines, with different doses of antigen with or without adjuvants were used in all the 10 studies. The dose of the vaccine antigen was defined on the basis of HA in 8 trials25,29,32–37 and on the basis of the virus surface proteins (HA and NA) in 2 trials.30,31 All adjuvanted vaccines elicited efficient antibody responses in elderly adults after two immunizations, with the SCR ranging from 33%-88%.25,29–33,35-37
Previous results, in contrast to the seasonal influenza vaccines, evidenced that H5N146,47 and H7N948,49 avain influenza virus vaccines have exhibited poor immunogenicity against HA. At least two doses of vaccine are required to elicit a robust immune response to the novel HA. In our meta-analysis, the results demonstrated that the SCR and SPR resulting from two doses of H5N1 vaccines in elderly adults were higher than those resulting from a single dose. Older adults vaccinated with two doses of OE-adjuvanted vaccines exhibited efficient seroconversion in all dose groups. Most of the included studies described inefficient antibody responses in a single dose of inactivated H5N1 vaccines and did not meet the CBER and CHMP criteria. However, the clinical trial conducted by Heijmans et al.35 described an SCR of 45% resulting from a single dose of OE-adjuvanted vaccine containing 3.75 µg of HA, whereas 3.75 µg of HA+NA elicited an SCR of 17% in the trial by Czajka et al.30 The inefficient antibody response to OE-adjuvanted vaccines may be due to insufficient HA content. These results suggest that two doses may be necessary for elderly adults. To investigate the optimal doses of H5N1 vaccines in elderly adults, we analyzed the differences in the antibody responses to two-dose of adjuvanted H5N1 vaccines. Our results showed that two doses of 7.5 µg of OE-adjuvanted H5N1 vaccines result in higher antibody response than 3.75 µg group, while no difference was found between 7.5 µg and 15 µg group. These results indicate that 7.5 µg may be the optimal dose for the H5N1 vaccines in elderly adults.
To generate a strong immune response to an influenza vaccine, an adjuvant is usually added to enhance the immunogenicity of the antigens in vivo.50–52 Although alum, MF59 and AS03 were the adjuvants used in 9 of the 10 studies included in this review, only two studies had control groups that were given unadjuvanted vaccines.25,35 The AS03-adjuvanted vaccine showed stronger antibody responses than the unadjuvanted vaccine in older adults, with high SCR (>70%) for two doses of AS03-adjuvanted vaccines.35 To investigate the effects of different adjuvants on the immunogenicity of H5N1 vaccines, we analyzed the consolidated SCR and SPR resulting from two doses of adjuvanted vaccines. The 7.5 μg and 15 μg of MF59-adjuvaned vaccines and all doses of AS03-adjuvanted vaccines induced robust antibody responses in elderly adults and met the CHMP or CBER criteria, whereas unadjuvanted vaccines and alum-adjuvanted vaccines showed low antibody responses in 3.75 μg, 7.5 μg and 15 μg groups, and did not meet the two vaccine production licensing criteria. Alum-adjuvanted inactivated influenza vaccines have demonstrated variable results, with effects ranging from moderate to none.53–55 In this review, a study conducted by Brady25 showed an SCR of 8% after two immunizations with 3.75 µg of an alum-adjuvanted vaccine, whereas Vajo et al.36 described an SCR of 60.7% that resulted from vaccination with 3.5 µg of alum-adjuvanted vaccine.
Studies with seasonal influenza vaccines suggest that the decreased immune response in elderly adults is partly due to immunosenescence. It is possible that elderly populations may therefore need additional or higher doses. To determine whether there is a lower immune response after H5N1 vaccination in elderly adults, we analyzed the differences in the SCR and SPR resulting from two doses of OE-adjuvanted H5N1 vaccines between younger and elderly adults. Slightly lower antibody response in elderly adults was found only in the group of 7.5 μg of OE-adjuvanted H5N1 vaccines when compared to younger adults.
Several limitations in our meta-analysis are worthy of mention. First, the composition of the vaccine can influence the antibody response. However, subgroup analysis for the whole virus, split-virion and subunit vaccines was not performed due to a limited number of studies. Second, only five studies did describe a prospective calculation of the scale of the study.30,32,34,35,37 Insufficient sample sizes may influence statistical conclusions to some extent. Third, the best way to evaluate the effectiveness of vaccines is usually the virus infection rate, hospitalization rate and infection-related mortality rate after immunization. However, none of the included studies mentioned the virus infection rate, hospitalization rate or infection-related mortality rate. Only the serological indicators SCR and SPR were used to analyze the protective effects of vaccines in this study. Finally, only clinical studies in English and Chinese were considered; thus, potential studies in other languages were not included.
In conclusion, we found that OE (MF59 or AS03)-adjuvaned H5N1 vaccines resulted in strong antibody responses in elderly adults. Furthermore, in our study, the H5N1 vaccine was well tolerated. Local and systemic adverse reactions were mild and self-limiting. Two doses of 7.5 µg of inactivated OE-adjuvanted H5N1 vaccines may be recommended for elderly adults. However, the composition of the vaccine can influence antibody responses. The effect of the vaccine type, such as adjuvanted vs. non-adjuvanted and inactivated whole virus vs. recombinant proteins, on the immune responses to H5N1 vaccines needs further clarification by way of large trials.
Acknowledgments
We would like to thank Dr. Yuanzhong Zhou, School of Public Health of Zunyi Medical University and Dr. Zheng Xiao, Department of Evidence-Based Medicine of the Affiliated Hospital of Zunyi Medical University for helpful discussion.
Funding Statement
This work was supported by the National Natural Science Foundation of China [no. 81860376].
Author contributions
KZ, XW and JH designed the study. KZ, XW, YS and XG developed the search strategy, searched the databases, screened the retrieved studies for eligibility and extracted the information from the studies. KZ and XW performed the data analysis. XW, KZ and JH wrote the original draft, which was reviewed and edited by JH. All authors approved the final written manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Centers for Disease Control and Prevention (CDC) . Isolation of avian influenza A(H5N1) viruses from humans–Hong Kong, May-December 1997. MMWR Morb Mortal Wkly Rep. 1997;46(50):1204–07. [PubMed] [Google Scholar]
- 2.Chan PKS. Outbreak of avian influenza A (H5N1) virus infection in Hong Kong in 1997. Clin Infect Dis. 2002;34:S58–64. doi: 10.1086/338820. [DOI] [PubMed] [Google Scholar]
- 3.Smith GJD, Wang J, Fan XH, Qin K, Zhang JX, Vijaykrishna D, Cheung CL, Rayner MJ, J Peiris JSM, Chen H, et al. Emergence and predominance of an H5N1 influenza variant in China. Proc Natl Acad Sci U S A. 2006;103(45):16936–41. doi: 10.1073/pnas.0608157103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shinya K, Makino A, Hatta M, Watanabe S, Kim JH, Hatta Y, Gao P, Ozawa M, Le QM, Kawaoka Y. Subclinical brain injury caused by H5N1 influenza virus infection. J Virol. 2011;85(10):5202–07. doi: 10.1128/JVI.00239-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO . Update: WHO-confirmed human cases of avian Influenza A (H5N1) infection, november 2003-May 2008. Wkly Epidemiol Rec. 2008;83(46):415–20. [PubMed] [Google Scholar]
- 6.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354(13):1343–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 7.Manzoli L, Salanti G, De Vito C, Boccia A, Ioannidis JPA, Villari P. Immunogenicity and adverse events of avian influenza A H5N1 vaccine in healthy adults: multiple-treatments meta-analysis. Lancet Infect Dis. 2009;9(8):482–92. doi: 10.1016/S1473-3099(09)70153-7. [DOI] [PubMed] [Google Scholar]
- 8.Guo Q, Liu Z, Gao J, Zhou J, Hu W, Cun Y, Li W, Liao G. Immunogenicity and safety of pandemic Influenza H5N1 vaccines in healthy adults through meta-analysis. Cell Physiol Biochem. 2016;40(5):921–32. doi: 10.1159/000453150. [DOI] [PubMed] [Google Scholar]
- 9.Banzhoff A, Nacci P, Podda A. A new MF59-adjuvanted influenza vaccine enhances the immune response in the elderly with chronic diseases: results from an immunogenicity meta-analysis. Gerontology. 2003;49(3):177–84. doi: 10.1159/000069172. [DOI] [PubMed] [Google Scholar]
- 10.Lui KJ, Kendal AP. Impact of influenza epidemics on mortality in the United States from October 1972 to May 1985. Am J Public Health. 1987;77(6):712–16. doi: 10.2105/ajph.77.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan TC, Hung IF, Cheng VC, Luk JK, Chu LW, Chan FH. Is nursing home residence an independent predictor of recurrent hospitalization in older adults? J Am Med Dir Assoc. 2013;14(9):706–07. doi: 10.1016/j.jamda.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. Jama. 2003;28(2):179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 13.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. Jama. 2004;292(11):1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 14.Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis. 2007;7(10):658–66. doi: 10.1016/S1473-3099(07)70236-0. [DOI] [PubMed] [Google Scholar]
- 15.Gasparini R, Lucioni C, Lai P, Maggioni P, Sticchi L, Durando P, Morelli P, Morelli P, Comino I, Calderisi S, et al. Cost-benefit evaluation of influenza vaccination in the elderly in the Italian region of Liguria. Vaccine. 2002;20(Suppl 5):B50–4. doi: 10.1016/s0264-410x(02)00507-8. [DOI] [PubMed] [Google Scholar]
- 16.Loperto I, Andrea S, Antonio N, Triassi M. Use of adjuvanted trivalent influenza vaccine in older-age adults: a systematic review of economic evidence. Hum Vaccin Immunother. 2019;15(5):1035–47. doi: 10.1080/21645515.2019.1578597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaffner W, Chen WH, Hopkins RH, Neuzil K. Effective immunization of older adults against seasonal influenza. Am J Med. 2018;131(8):865–73. doi: 10.1016/j.amjmed.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Rumke HC, Richardus JH, Rombo L, Pauksens K, Plaßmann G, Durand C, Devaster JM, Dewé W, Oostvogels L. Selection of an adjuvant for seasonal influenza vaccine in elderly people: modelling immunogenicity from a randomized trial. BMC Infect Dis. 2013;13:348. doi: 10.1186/1471-2334-13-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaffner W, van Buynder P, McNeil S, Osterhaus ADEM. Seasonal influenza immunisation: strategies for older adults. Int J Clin Pract. 2018;72:e13249. doi: 10.1111/ijcp.13249. [DOI] [PubMed] [Google Scholar]
- 20.Weinberger B. Adjuvant strategies to improve vaccination of the elderly population. Curr Opin Pharmacol. 2018;41:34–41. doi: 10.1016/j.coph.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Cancro MP, Hao Y, Scholz JL, Riley RL, Frasca D, Dunn-Walters KD, Blomberg BB. B cells and aging: molecules and mechanisms. Trends Immunol. 2009;30(7):313–18. doi: 10.1016/j.it.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell K, Chung JR, Monto AS, Martin ET, Belongia EA, McLean HQ, Gaglani M, Murthy K, Zimmerman RK, Nowalk MP, et al. Influenza vaccine effectiveness in older adults compared with younger adults over five seasons. Vaccine. 2018;36(10):1272–78. doi: 10.1016/j.vaccine.2018.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(1):36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 24.Sobhanie M, Matsuoka Y, Jegaskanda S, Fitzgerald T, Mallory R, Chen Z, Luke C, Treanor J, Subbarao K. Evaluation of the safety and immunogenicity of a candidate pandemic live attenuated influenza vaccine (pLAIV) against influenza A(H7N9). J Infect Dis. 2016;213(6):922–29. doi: 10.1093/infdis/jiv526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brady RC, Treanor JJ, Atmar RL, Keitel WA, Edelman R, Chen WH, Winokur P, Belshe R, Graham IL, Noah DL, et al. Safety and immunogenicity of a subvirion inactivated influenza A/H5N1 vaccine with or without aluminum hydroxide among healthy elderly adults. Vaccine. 2009;27(37):5091–95. doi: 10.1016/j.vaccine.2009.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gou X, Xiaoxue W, Shi Y, Zhang K, Huang J. A systematic review and meta-analysis of cross-reactivity of antibodies induced by H7 influenza. Hum Vaccin Immunother. 2020;16(2):286–94. doi: 10.1080/21645515.2019.1649551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guideline on dossier structure and content for pandemic influenza vaccine marketing authorisation application (revision).Committee for guidance for human medicinal products (CHMP). EMEA/CPMP/VEG/4717/2003- Rev. 1; 2008.
- 28.Guidance for industry: clinical data needed to support the licensure of pandemic influenza vaccines. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research (CBER); 2007. [Google Scholar]
- 29.Leroux-Roels I, Van der Wielen M, Kafeja F, Vandermeulen C, Lazarus R, Snape MD, John T, Carre C, Nougarede N, Pepin S, et al. Humoral and cellular immune responses to split-virion H5N1 influenza vaccine in young and elderly adults. Vaccine. 2009;27(49):6918–25. doi: 10.1016/j.vaccine.2009.08.110. [DOI] [PubMed] [Google Scholar]
- 30.Czajka H, Unal S, Ulusoy S, Usluer G, Strus A, Sennaroglu E, Guzik J, Iskit AT, Dargiewicz A, Musial D, et al. A phase II, randomised clinical trial to demonstrate the non-inferiority of low-dose MF59-adjuvanted pre-pandemic A/H5N1 influenza vaccine in adult and elderly subjects. J Prev Med Hyg. 2012;53(3):136–42. [PubMed] [Google Scholar]
- 31.Vesikari T, Forsten A, Herbinger KH, Cioppa GD, Beygo J, Borkowski A, Groth N, Bennati M, Sonnenburg FV. Safety and immunogenicity of an MF59((R))-adjuvanted A/H5N1 pre-pandemic influenza vaccine in adults and the elderly. Vaccine. 2012;30(7):1388–96. doi: 10.1016/j.vaccine.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Banzhoff A, Gasparini R, Laghi-Pasini F, Staniscia T, Durando P, Montomoli E, Capecchi PL, Giovanni PD, Sticchi L, Gentile C, et al. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS One. 2009;4(2):e4384. doi: 10.1371/journal.pone.0004384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bihari I, Panczel G, Kovacs J, Beygo J, Fragapane E. Assessment of antigen-specific and cross-reactive antibody responses to an MF59-adjuvanted A/H5N1 prepandemic influenza vaccine in adult and elderly subjects. Clin Vaccine Immunol. 2012;19(12):1943–48. doi: 10.1128/CVI.00373-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MVM VDV, Aichinger G, Pollabauer EM, Löw-Baselli A, Fritsch S, Benamara K, Kistner O, Müller M, Zeitlinger M, Kollaritsch H, et al. Cell culture (Vero cell) derived whole-virus non-adjuvanted H5N1 influenza vaccine induces long-lasting cross-reactive memory immune response: homologous or heterologous booster response following two dose or single dose priming. Vaccine. 2012;30(43):6127–35. doi: 10.1016/j.vaccine.2012.07.077. [DOI] [PubMed] [Google Scholar]
- 35.Heijmans S, De Meulemeester M, Reynders P, Giet D, Demanet E, Devresse PY, Icardi G, Dramé M, Roman F, Gillard P. Immunogenicity profile of a 3.75-mug hemagglutinin pandemic rH5N1 split virion AS03A-adjuvanted vaccine in elderly persons: a randomized trial. J Infect Dis. 2011;203(8):1054–62. doi: 10.1093/infdis/jiq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vajo Z, Wood J, Kosa L, Szilvasy I, Paragh G, Pauliny Z, Bartha K, Visontay I, Kis A, Jankovics I. A single-dose influenza A (H5N1) vaccine safe and immunogenic in adult and elderly patients: an approach to pandemic vaccine development. J Virol. 2010;84(3):1237–42. doi: 10.1128/JVI.01894-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.S E F, Shakib S, Chanthavanich P, Richmond P, Smith T, Tantawichien T, Kittel C, Jaehnig P, ZSafety M, Kanesa-Thasan N, et al. Safety and immunogenicity of MF59-adjuvanted cell culture–derived A/H5N1 subunit influenza virus vaccine: dose-finding clinical trials in adults and the elderly. Open Forum Infect Dis. 2019;6(4):ofz107. doi: 10.1093/ofid/ofz107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cordero E, Roca-Oporto C, Bulnes-Ramos A, Aydillo T, Gavaldà J, Moreno A, Torre-Cisneros J, Montejo JM, Fortun J, Muñoz P, et al. Two doses of inactivated influenza vaccine improve immune response in solid organ transplant recipients: results of TRANSGRIPE 1-2, a randomized controlled clinical trial. Clin Infect Dis. 2017;64(7):829–38. doi: 10.1093/cid/ciw855. [DOI] [PubMed] [Google Scholar]
- 39.Coughlan L, Sridhar S, Payne R, Edmans M, Milicic A, Venkatraman N, Lugonja B, Clifton L, Qi C, Folegatti PM, et al. Heterologous two-dose vaccination with simian adenovirus and poxvirus vectors elicits long-lasting cellular immunity to influenza virus A in healthy adults. EBioMedicine. 2018;29:146–54. doi: 10.1016/j.ebiom.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ko EJ, Kang SM. Immunology and efficacy of MF59-adjuvanted vaccines. Hum Vaccin Immunother. 2018;14(12):3041–45. doi: 10.1080/21645515.2018.1495301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Del Giudice G, Rappuoli R, Didierlaurent AM. Correlates of adjuvanticity: a review on adjuvants in licensed vaccines. Semin Immunol. 2018;39:14–21. doi: 10.1016/j.smim.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Gemmill I, Young K. Summary of the NACI literature review on the comparative effectiveness of subunit and split virus inactivated influenza vaccines in older adults. Can Commun Dis Rep. 2019;45(6):129–33. doi: 10.14745/ccdr.v44i06a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tambyah PA, Wilder-Smith A, Pavlova BG, Barrett PN, Oh HML, Hui DS, Yuen KY, Fritsch S, Aichinger G, Loew-Baselli A, et al. Ehrlich. Safety and immunogenicity of two different doses of a Vero cell-derived, whole virus clade 2 H5N1 (A/Indonesia/05/2005) influenza vaccine. Vaccine. 2012;30(2):329–35. doi: 10.1016/j.vaccine.2011.10.088. [DOI] [PubMed] [Google Scholar]
- 44.Treanor JJ, Essink B, Hull S, Reed S, Izikson R, Patriarca P, Goldenthal KL, Kohberger R, Dunkle LM. Evaluation of safety and immunogenicity of recombinant influenza hemagglutinin (H5/Indonesia/05/2005) formulated with and without a stable oil-in-water emulsion containing glucopyranosyl-lipid A (SE + GLA) adjuvant. Vaccine. 2013;31(48):5760–65. doi: 10.1016/j.vaccine.2013.08.064. [DOI] [PubMed] [Google Scholar]
- 45.Dermont MA, Elmer T. Influenza syndromic surveillance and vaccine efficacy in the UK armed forces, 2017-2018. J R Army Med Corps. 2019;165(6):395–99. doi: 10.1136/jramc-2018-001067. [DOI] [PubMed] [Google Scholar]
- 46.Belshe RB, Frey SE, Graham IL, Edwin L, Anderson EL, Jackson LA, Spearman P, Edupuganti S, Mulligan MJ, Rouphael N, et al. Immunogenicity of avian influenza A/Anhui/01/2005(H5N1) vaccine with MF59 adjuvant: a randomized clinical trial. Jama. 2014;312(14):1420–28. doi: 10.1001/jama.2014.12609. [DOI] [PubMed] [Google Scholar]
- 47.Chen WH, Jackson LA, Edwards KM, Keitel WA, Hill H, Noah DL, Creech CB, Patel SM, Mangal B, Kotloff KL. Safety, reactogenicity, and immunogenicity of inactivated monovalent influenza A (H5N1) virus vaccine administered with or without AS03 adjuvant. Open Forum Infect Dis. 2014;1(3):ofu091. doi: 10.1093/ofid/ofu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu UI, Hsieh SM, Lee WS, Wang NC, Kung HC, Ou TY, Chen FL, Lin TY, Chen YC, Chang SH. Safety and immunogenicity of an inactivated cell culture-derived H7N9 influenza vaccine in healthy adults: A phase I/II, prospective, randomized, open-label trial. Vaccine. 2017;35(33):4099–104. doi: 10.1016/j.vaccine.2017.06.044. [DOI] [PubMed] [Google Scholar]
- 49.Mulligan MJ, Bernstein DI, Winokur P, Rupp R, Anderson E, Rouphael N, Dickey M, Stapleton JT, Edupuganti S, Spearman P, et al. Serological responses to an avian influenza A/H7N9 vaccine mixed at the point-of-use with MF59 adjuvant: a randomized clinical trial. Jama. 2014;312(14):1409–19. doi: 10.1001/jama.2014.12854. [DOI] [PubMed] [Google Scholar]
- 50.Banzhoff A, Pellegrini M, Del Giudice G, Fragapane E, Groth N, Podda A. MF59-adjuvanted vaccines for seasonal and pandemic influenza prophylaxis. Influenza Other Respir Viruses. 2008;2(6):243–49. doi: 10.1111/j.1750-2659.2008.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diallo A, Victor JC, Feser J, Ortiz JR, Kanesa-Thasan N, Ndiaye M, Diarra B, Cheikh S, Diene D, Ndiaye T, et al. Immunogenicity and safety of MF59-adjuvanted and full-dose unadjuvanted trivalent inactivated influenza vaccines among vaccine-naive children in a randomized clinical trial in rural Senegal. Vaccine. 2018;36(43):6424–32. doi: 10.1016/j.vaccine.2018.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang WT, Huang YS, Hsu CY, Chen HC, Lee HC, Lin HC, Hsieh CF, Wu MN, Yang CH. Narcolepsy and 2009 H1N1 pandemic vaccination in Taiwan. Sleep Med. 2018;66:276–81. doi: 10.1016/j.sleep.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 53.Basavaraj VH, Sampath G, Hegde NR, Mohan VK, Ella KM. Evaluation of safety and immunogenicity of HNVAC, an MDCK-based H1N1 pandemic influenza vaccine, in phase I single centre and phase II/III multi-centre, double-blind, randomized, placebo-controlled, parallel assignment studies. Vaccine. 2014;32(35):4592–97. doi: 10.1016/j.vaccine.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 54.Keitel WA, Dekker CL, Mink C, Campbell JD, Edwards KM, Patel SM, Ho DY, Talbot HK, Guo K, Noah DL, et al. Safety and immunogenicity of inactivated, Vero cell culture-derived whole virus influenza A/H5N1 vaccine given alone or with aluminum hydroxide adjuvant in healthy adults. Vaccine. 2009;27(47):6642–48. doi: 10.1016/j.vaccine.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin DP, Zhu BP, Wang HQ, Cao L, Wu WD, Jiang KY, Xia W, Guo Zhang M, Zheng JS, Cao LS, et al. Effect of aluminum hydroxide adjuvant on the immunogenicity of the 2009 pandemic influenza A/H1N1 vaccine: multi-level modeling of data with repeated measures. Biomed Environ Sci. 2011;24(6):624–29. doi: 10.3967/0895-3988.2011.06.006. [DOI] [PubMed] [Google Scholar]


