INTRODUCTION:
Guidelines for surveillance after polypectomy are lacking in strong evidence. Our aim was to identify some precursors of colorectal cancer lesions at 3 years after polypectomy to improve stratification and surveillance programs.
METHODS:
We included patients with high-risk lesions (HRLs), defined as advanced adenoma (AA), large serrated polyps (SPs), and multiplicity (≥3 of any adenomas/SPs). Data on age, sex, cardiovascular risk factors, pharmacological treatment, and the histological characteristics in each individual, and mutations in genes involved in the most advanced index polyp, were collected. Parameters independently associated with a metachronous HRL diagnosis were evaluated through univariate and multivariate analyses. The results are reported as odds ratios and 95% confidence intervals along with P values.
RESULTS:
A total of 537 cases (median age: 60.7 years; 66% male) were included. Dyslipidemia and smoking correlated with metachronous HRLs. Multivariate logistic regression analysis showed that the presence of multiplicity with ≥3 polyps on the index colonoscopy was significantly associated with metachronous HRL, AA, proximal AA, and ≥3 polyps at 3 years. In addition, independent predictors of metachronous proximal AA were increasing age, female sex, and the loss of expression of the MLH1 protein.
DISCUSSION:
Multiplicity was a strong predictor of HRLs at 3 years, although the inclusion of other clinical variables (age, sex, smoking status, and dyslipidemia) improves surveillance recommendations. Without these risk factors, the surveillance could be extended to 5 years; we propose examining the somatic expression of MHL1 in all patients.
INTRODUCTION
Advanced adenomas (AAs), defined as having more than 25% villous histology and/or a size ≥10 mm, and/or high-grade dysplasia, are the main precursor of colorectal cancer (CRC) (1,2). Moreover, large serrated polyps (SPs) (≥10 mm) are also considered equally premalignant lesions responsible for approximately 15% of CRC cases (3,4). Endoscopic polypectomy reduces the incidence and mortality of CRC and remains the key to successful population screening programs (2,5,6). Recent evidence confirms that individuals with AA and/or large SPs have a 3–4 times higher risk of mortality due to CRC than individuals without polyps (7,8). The risk of developing new lesions over time is associated with different factors (9,10). Individuals with low-risk adenomas (LRAs) (1–2 in number, tubular, <10 mm in size, and with low-grade dysplasia) diagnosed during their first colonoscopy have a 6.9% AA risk after 5 years from the procedure, whereas those with high-risk adenomas (HRAs) (AA and/or ≥3 synchronous adenomas) have a 15.5% risk after 5 years from the procedure (11). One analysis from a population-based colonoscopy registry showed that patients observed with large SPs at index colonoscopy had an increased risk of metachronous large SPs, and those with both HRAs and SPs had a considerably increased risk of metachronous HRAs (12). Colonoscopy remains the main strategy for surveillance after index polypectomy. The intervals between colonoscopies are based on the size, number, and histopathology of the removed polyps. However, the current method of risk stratification is not accurate enough because of the lack of strong evidence (7,8,13,14).
Some research has pointed to other factors significantly related to metachronous lesions, such as a family history of CRC, sedentary lifestyle, smoking, and cardiovascular risk factors of metabolic syndrome (MetS) (15–17). Furthermore, some somatic molecular polyp characteristics have been linked to metachronous lesions. It is well known that molecular alterations occur during the progression of the adenoma-carcinoma sequence, which disturbs the cellular homeostasis. It has been postulated that these alterations can be a predisposition to new neoplasm development (18,19). In fact, the existence of the KRAS somatic mutation has been shown to be related to AA recurrences (20,21). For all of these reasons, applying the integrative concept of the “etiological field effect,” which indicates that the interaction of multiple etiological and exogenous factors contribute to the initiation and progression of neoplasia, could help to improve the precision of risk stratification (22).
The goal of surveillance after polypectomy was to identify metachronous high-risk lesions (HRLs), which are defined as AA, large SPs, and/or having multiplicity (≥3 adenomas and/or SPs), for use as surrogate markers to signal the future risk of CRC (12). Thus, knowing the predictive factors of metachronous HRLs is essential and will improve stratification and surveillance programs.
Our aim was to identify the predictive factors for metachronous HRLs at 3 years after polypectomy. The study was conducted on a cohort drawn from a population-based CRC screening program at the index colonoscopy. We incorporated an evaluation of the environmental and clinical factors and the histopathological and somatic molecular changes based on the most advanced index polyp.
METHODS
We conducted a single-center retrospective study on a cohort that had an average risk of CRC (asymptomatic individuals between 50 and 69 years of age) from the Barcelona CRC Screening Program 2009–2011. The selected individuals had all been diagnosed with HRL through colonoscopy after a fecal immunochemistry test (OC-sensor ≥20 μg Hb/g of feces) and who had undergone surveillance colonoscopy at 3 years ± 6 months after polypectomy. We recorded which cases underwent more than one colonoscopy to ensure complete resection of polyps.
Informed consent was obtained from all patients. The biological samples were obtained from Barcelona Parc de Salut MAR Biobank (MARBiobanc), and the study was approved by the Hospital del Mar Clinical Research Ethics Committee (Ref. 2016S004). All colonoscopies fulfilled the standard quality policy (23), and all endoscopists adhered to the recommended adenoma detection rate standards.
Individuals with a personal and/or familial history of CRC or adenoma, inflammatory bowel disease, incomplete histopathological analysis of recovered polyps, and individuals who did not provide informed consent were excluded. Individuals who were observed to have SPs exclusively at the index colonoscopy were excluded because the number of SPs in such individuals was low.
Clinical and pathological data
We assessed the data on age, sex, the fecal immunochemistry test before colonoscopy, body mass index, smoking history, high blood pressure (25), type 2 diabetes mellitus, and dyslipidemia, defined as elevated plasma cholesterol, triglycerides, or both, or low HDL cholesterol. Data on the presence of MetS, as defined by the World Health Organization (26), administration of aspirin, nonsteroidal anti-inflammatory drugs, and statins and on any chronic treatment were also collected. Furthermore, the number of polyps, their size, location (distal or proximal to the splenic flexure), and histopathology at the basal and the surveillance colonoscopies were recorded.
Molecular markers
The molecular study was performed on the most advanced histological polyp in every patient. Two experienced pathologists selected the relevant section of the paraffin-embedded samples.
The genes most commonly involved in colorectal carcinogenesis that have been linked to the risk of advanced or metachronous polyps were studied based on the pathway, chromosomes, or microsatellite instabilities. These genes were KRAS, NRAS, BRAF, APC, TP53, FBXW7, CTNNB1, SMAD4, Ki-67, MLH-1, CYTOKERATIN 7, CYTOQUERATIN 20, and CDX2. DNA was extracted from formalin-fixed, paraffin-embedded blocks of the sample using the MagCore Genomic DNA FFPE One-Step Kit—MagCore HF 16 Plus (24,25).
Sequencing of the somatic mutation study.
A sample library was generated with a QIAseq Targeted DNA panel (Qiagen) containing the coding region of the genes (see Supplementary Digital Contents 1 and 2, Supplementary Tables 1, http://links.lww.com/CTG/A498, and http://links.lww.com/CTG/A499). The resulting library was sequenced on the next-generation sequencing platform, MiSeq (Illumina, San Diego, CA), and analyzed with the QIASeq DNA pipeline. Variants obtained were filtered and annotated with Variant Studio v3.0 and visualized with Integrative Genomics Viewer v2.4. The average depth of coverage was 1559 x. The frequent CRC-associated variants in KRAS, BRAF, NRAS, APC, and TP53 genes with variant allelic frequency <5% were considered true variants. Those variants with a variant allelic frequency >5% were considered less frequent variants to minimize the probability of false positive results.
All mutations identified were verified against a catalogue of somatic mutations in the cancer database and VarSome (27). The mutation nomenclature used follows the Human Genome Variation Society's recommendations.
Immunohistochemistry technique
Tissue microarray construction.
Representative hematoxylin-eosin stained sections from polyps were prepared by 2 experienced gastrointestinal pathologists. The most histopathologically advanced polyp area was selected for the construction of the tissue microarray (TMA) blocks using a tissue arrayer. Each case was represented in the final TMA paraffin block by 2 tissue cores, each being 1.5 mm in diameter.
Immunohistochemistry tests were performed on the formalin-fixed paraffin-embedded TMA blocks. Sections were cut at 4 μm and then dewaxed and rehydrated. We used an automatized panel of Roche Ventana antibodies (Benchmarkt) (see Supplementary Digital Contents 1 and 2, Supplementary Tables 1, http://links.lww.com/CTG/A498, and 2 http://links.lww.com/CTG/A499). Each marker was semiquantitatively assessed and scored by estimating the percentage of tumor cells showing characteristic staining.
Membrane staining for CK7 and CK20 and nuclear staining for CDX-2 were considered positive when more than 5% of the tumor cells showed a positive reaction for each marker; Ki-67 was considered positive when more than 5% of the nonbasal located cells showed nuclear staining irrespective of intensity (28,29). Loss of MLH1 expression was considered when one or more clusters of tumor cells (minimal, focal, or multifocal) or all dysplastic cells showed ≥50% non-nuclear staining, compared with positive nuclear staining in normal epithelial cells (30).
Statistical analysis
Differences between demographic, clinical, and molecular data among patients with and without metachronous advanced lesions were tested with the χ2 test. Multivariate logistic regression analysis was conducted to identify independent parameters associated with metachronous HRLs. The results are expressed as odds ratios (ORs) with 95% confidence intervals (CIs). SPSS version 25 (IBM, Armonk, NY) was used for the analyses.
RESULTS
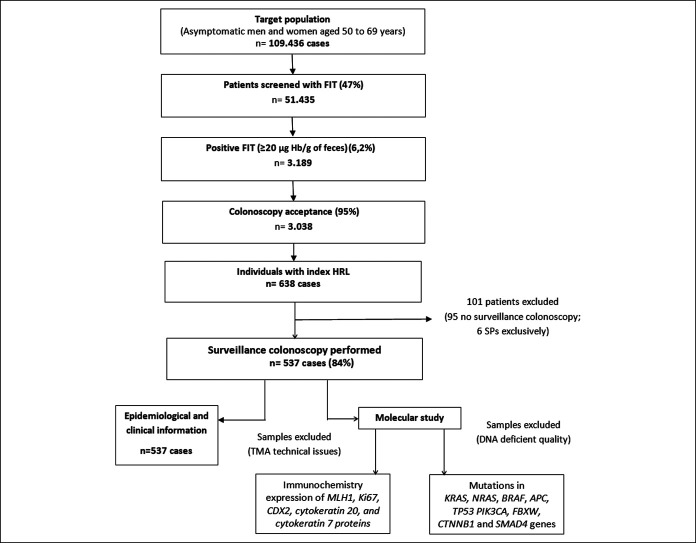
We identified 638 individuals with HRLs at the baseline colonoscopy. We excluded 95 cases (14.8%) lacking surveillance colonoscopy and 6 cases with exclusively index SPs. A total of 537 cases (85.2%) that met the inclusion and exclusion criteria (median age 60.7 ± 5.2 years; 66% men) were analyzed. The median surveillance colonoscopy time was 38.8 months, and the study flow chart is presented in Figure 1.
Figure 1.
Study flow chart. Patients included in the analysis. FIT, fecal immunochemical test; HRL, high-risk lesions; SP, serrated polyps; TMA, tissue microarray.
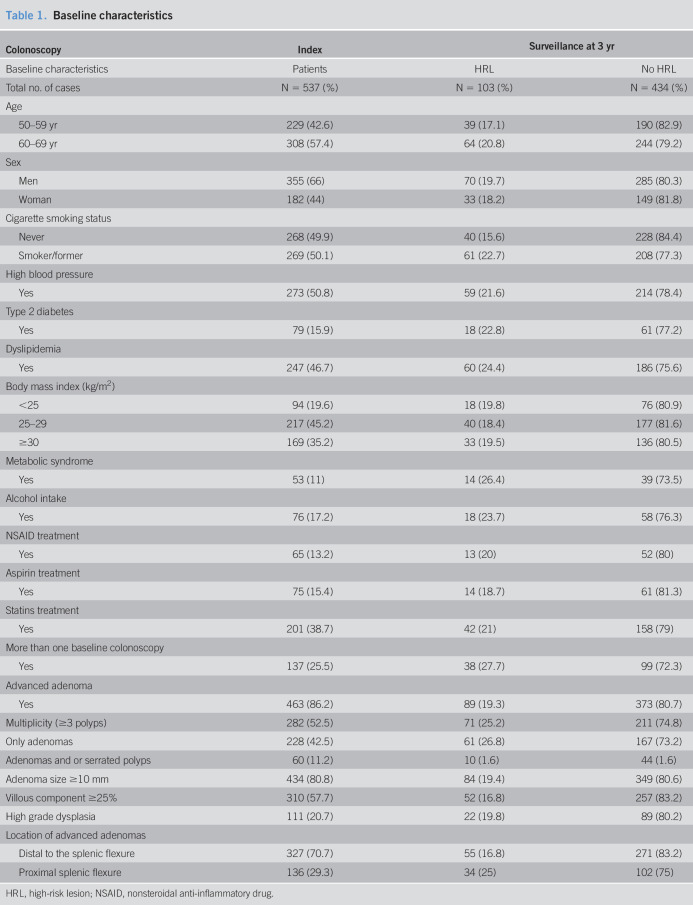
Characteristics of the cohort included in the study are presented in Table 1. Half of the study population had had some exposure to tobacco, and 47% had been diagnosed with dyslipidemia. Of note, nearly 80% of the included individuals were overweight or obese (Figure 2).
Table 1.
Baseline characteristics
HRL, high-risk lesion; NSAID, nonsteroidal anti-inflammatory drug.
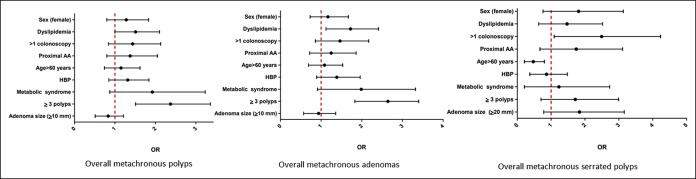
Figure 2.
Predictive factors of metachronous overall lesions. Multivariate analysis. AA, advanced adenoma; HBP, high blood pressure; OR, odds ratio.
To achieve complete polyp resection, 137 cases (25.2%) required more than one colonoscopy, most of them because of control of the piecemeal removed lesions (70%) or for complete resection of the remaining polyps (30%).
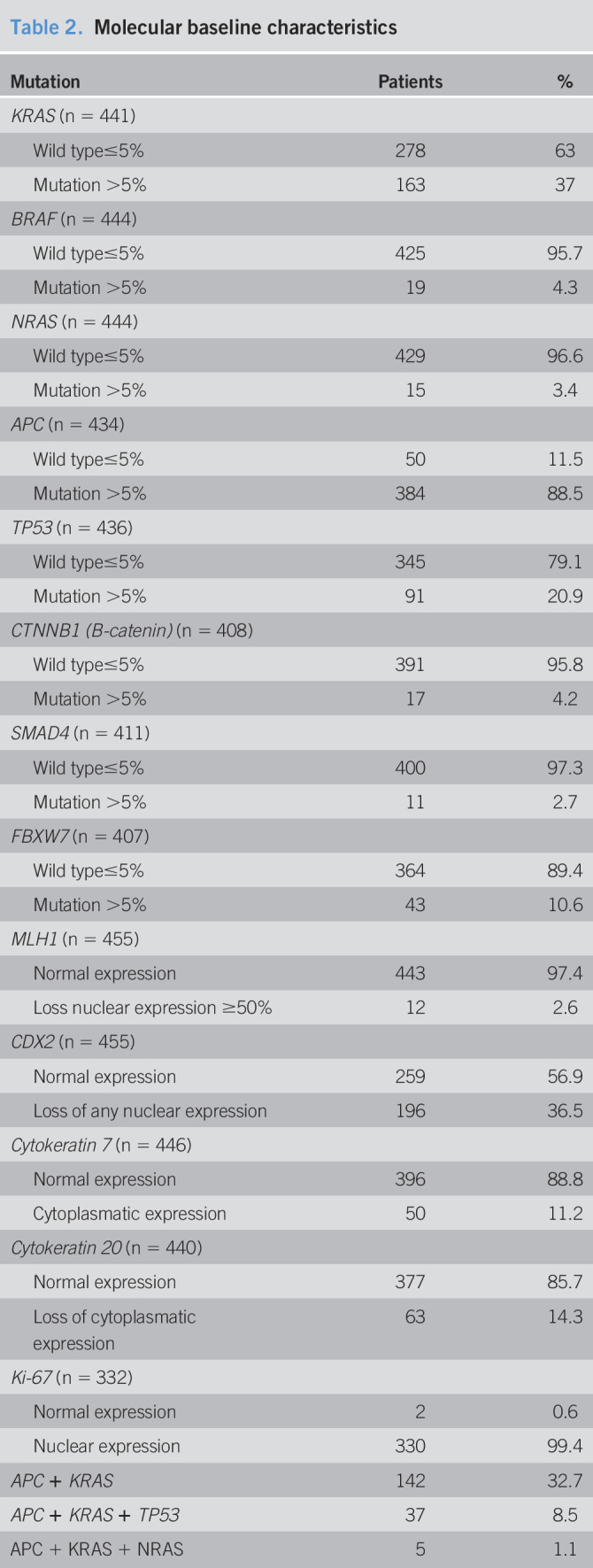
The somatic molecular findings for the most histologically advanced polyps in every individual are provided in Table 2. As expected, most of the AAs had mutations in the APC gene. KRAS mutations were more frequent in AAs (P < 0.05). In addition, although they were not too frequent, BRAF mutations and loss of MLH1 expression were found in proximal AAs. The mutation data for the genes analyzed are presented in Supplementary Tables 1 and 2 (see Supplementary Digital Contents 1 and 2, http://links.lww.com/CTG/A498, and http://links.lww.com/CTG/A499).
Table 2.
Molecular baseline characteristics
At surveillance colonoscopies, 103 cases (19%) were classified as HRLs. There were 54 patients with AAs (10.1%), with 29 of the AAs (5.4%) located in the proximal colon. In 73 cases (13.6%), we found multiplicity (63 cases with ≥3 adenomas and 10 cases with ≥3 adenomas plus large SPs). Nine patients (1.7%) had large SPs in the proximal colon. The remaining 435 individuals (81.0%) had normal colonoscopy findings at 3 years (48.9%) or had low-risk lesions (32.0%). No CRCs were diagnosed.
Risk of advanced lesions at the surveillance colonoscopy
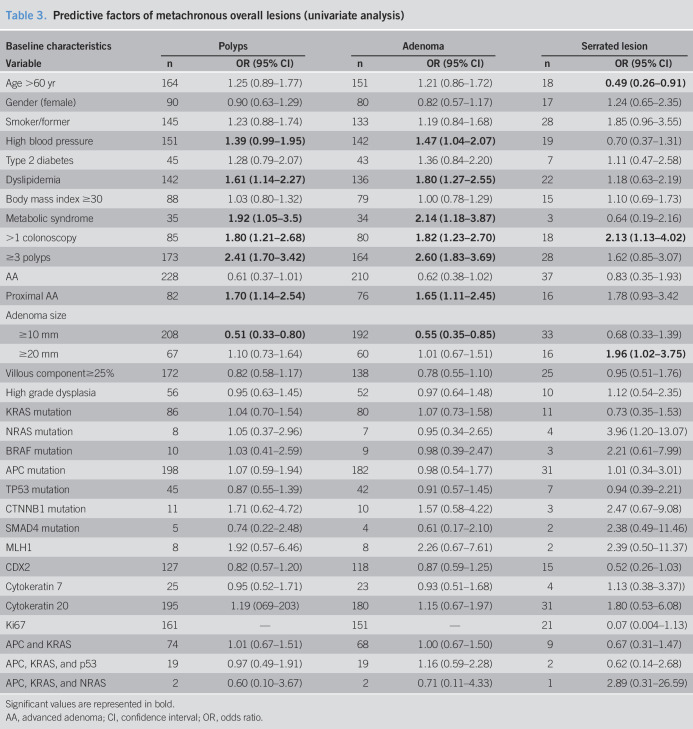
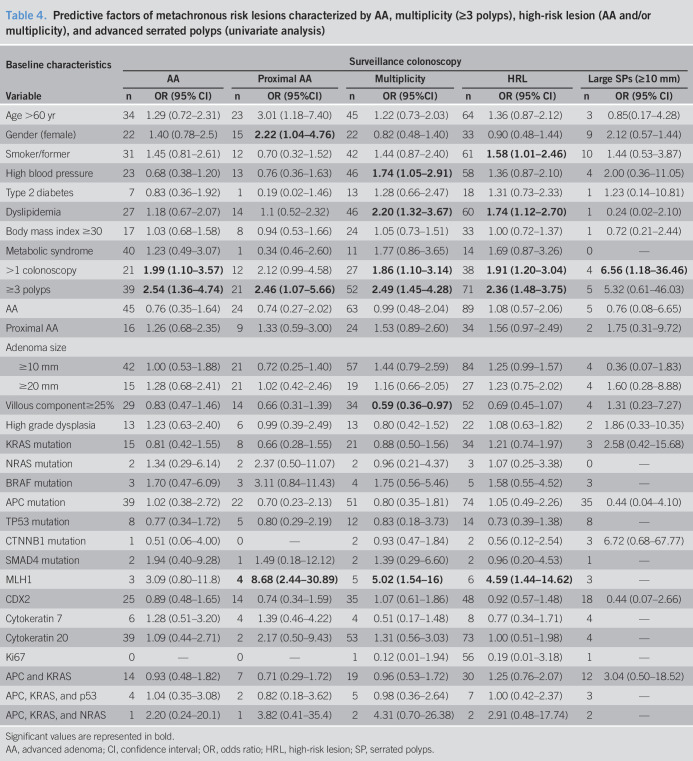
The univariate analysis results are reported in Tables 3 and 4. When we evaluated the clinical factors, the patients who were current or former smokers had a slightly but statistically significantly increased metachronous HRL risk (OR = 1.58, 95% CI: 1.01–2.46) as did those with dyslipidemia (OR = 1.74, 95% CI: 1.12–2.70). A history of dyslipidemia or high blood pressure correlated with multiplicity at the surveillance colonoscopy (OR = 2.20, 95% CI: 1.32–3.67; OR = 1.74, 95% CI: 1.05–2.91, respectively). In addition, those presenting with multiplicity at the index colonoscopy were more than twice as likely to develop metachronous AAs (OR = 2.54, 95% CI: 1.36–4.74), proximal AAs (OR = 2.46, 95% CI: 1.07–5.66), repeated multiplicity (OR = 2.49, 95% CI: 1.45–4.28), and HRLs (OR = 2.36, 95% CI: 1.48–3.75). The need for additional colonoscopies after the index colonoscopy correlated with increased occurrence of metachronous AAs, multiplicity, and HRLs (OR = 1.99, 95% CI: 1.10–3.57; OR = 1.86, 95% CI: 1.10–3.14; OR = 1.91, 95% CI: 1.20–3.04, respectively). Of all the studied baseline characteristics, large metachronous SPs were only significantly associated with the need for more than one index colonoscopy (OR = 6.56, 95% CI: 1.18–36.46), although the number of metachronous large SPs was small. We evaluated the mutations individually and in groups from the same polyp for the power to predict metachronous lesions at 3 years, which turned out to be poor in both cases. Loss of nuclear expression of the MLH1 gene at the time of baseline colonoscopy resulted in an 8-fold increased risk of proximal AAs (OR = 8.68, 95% CI: 2.44–30.89) and up to a 5-fold increased risk of multiplicity (OR = 5.02, 95% CI: 1.54–16) and HRLs (OR = 4.59, 95% CI: 1.44–14.62) at the follow-up colonoscopy.
Table 3.
Predictive factors of metachronous overall lesions (univariate analysis)
Significant values are represented in bold.
AA, advanced adenoma; CI, confidence interval; OR, odds ratio.
Table 4.
Predictive factors of metachronous risk lesions characterized by AA, multiplicity (≥3 polyps), high-risk lesion (AA and/or multiplicity), and advanced serrated polyps (univariate analysis)
Significant values are represented in bold.
AA, advanced adenoma; CI, confidence interval; OR, odds ratio; HRL, high-risk lesion; SP, serrated polyps.
Multivariate analysis
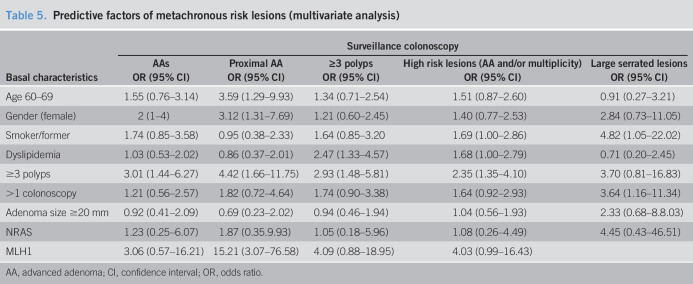
Multivariate logistic regression analysis (Table 5) confirmed that older individuals and women had a higher risk of being diagnosed with metachronous proximal AAs (OR = 3.59, 95% CI: 1.29–9.93; OR = 3.12, 95% CI: 1.31–7.69, respectively). Dyslipidemia and smoking history were associated with a higher risk of developing HRLs (OR = 1.68, 95% CI: 1.00–2.79; OR = 1.69, 95% CI: 1.00–2.86, respectively). Individuals with index multiplicity were 3 times more likely to be diagnosed at the 3-year surveillance of HRL (OR = 2.35, 95% CI: 1.35–4.10), AA (OR = 3.01, 95% CI: 1.44–6.27), proximal AA (OR = 4.42, 95% CI: 1.66–11.75), and multiplicity (OR = 2.93, 95% CI: 1.48–5.81) than those with no multiplicity. From the molecular somatic analysis, the multivariable analysis showed that the expression of the MLH1 protein was significantly associated with proximal AAs at surveillance (OR = 15.21, 95% CI: 3.07–76.58) and the mutation in NRAS was significantly associated with any size of SP at surveillance 4.42 (95% CI: 1.31–14.91).
Table 5.
Predictive factors of metachronous risk lesions (multivariate analysis)
AA, advanced adenoma; CI, confidence interval; OR, odds ratio.
DISCUSSION
This study confirms that multiplicity is a strong predictor of metachronous HRLs and also highlights the importance of factors such as a history of smoking, dyslipidemia, older age, female sex, and loss of MLH1 protein expression. These results reveal the need to optimize current surveillance guideline strategies.
At the 3-year follow-up, 19% of patients with HRL at the index colonoscopy had metachronous HRLs. These results were comparable with those of other studies (31). However, the variability in HRL recurrence in published studies is high because it depends not only on individual risk factors but also on the colonoscopy quality and the subsequent polyps' miss rate (MR) (9,32,33). In our cohort, we estimated a 13% MR for AAs, a proportion higher the 9% MR published in the meta-analysis by Zhao et al. (34). Our MR was likely overestimated because we obtained it from surveillance colonoscopies performed to ensure complete resection (46).
Older age and female sex were robust predictors of metachronous proximal AAs (OR = 3.59, 95% CI: 1.29–9.93; OR = 3.12, 95% CI: 1.31–7.69, respectively). Older age has been identified as an independent HRL risk factor (9,35), especially for HRLs in the proximal colon (36). This might be because of decreased immunity and genetic variations that occur with advancing age (35). The Clinical Outcomes Research Initiative endoscopy database provides the opportunity to study the differences in polyps and tumors with differences in age and sex. Using this, we found that women have a greater tendency of developing right-sided polyps. Indeed, hormonal factors may explain a large percentage of the metachronous right-sided AAs observed in women (37) because estrogen exposure is a protective factor against the Microsatellite Instable high phenotype, which often accompanies right-sided tumors (38). Therefore, it is necessary to emphasize that special attention must be given in the detection of proximal lesions in women older than the age of 60 years.
There is limited information about the role of each of the components of MetS in metachronous HRL development (16). Nevertheless, we found a higher proportion of new HRLs at surveillance colonoscopy in patients with dyslipidemia; up until now, the molecular mechanism behind this is not well understood (38,39).
Likewise, carcinogenic compounds in tobacco, mainly aromatic amines, can cause mutations in genes implicated in CRC, such as KRAS and BRAF (40). In a retrospective study, higher nicotine levels between the index and follow-up colonoscopies were correlated with metachronous colorectal neoplasia risk (41). These findings have important public health implications because they show that improving the plasma levels of cholesterol/triglycerides and giving up cigarette smoking would be effective measures for preventing colorectal metachronous polyps.
In our series, exposure to tobacco and the presence of dyslipidemia had a higher risk of global metachronous HRL, whereas older age, female sex, and loss of MLH1 expression were significantly associated with HRLs on the right side (36,42). Thus, our results could suggest that genetic factors have a greater effect on the proximal colon, whereas environmental factors have a more global effect (36,42).
Some characteristics found in index polyps are indicative of an increased risk of metachronous lesions. However, these findings are sufficiently helpful for planning early surveillance strategies, most likely because their effect could depend on latency time. We did not find any histological characteristics that increased metachronous HRL risk. Nonetheless, we report that multiplicity on index colonoscopy was a powerful predictor of HRL recurrence, possibly owing to genetic imbalance of cell proliferation in some individuals, which could lead to accelerated carcinogenesis on normal mucosa. Therefore, it is important to ensure that proper surveillance is performed on these individuals(43,44,46).
In addition, we found that NRAS mutations predicted the risk of SPs of any size 3 years after the polypectomy, and the loss of MLH1 protein expression was associated with proximal AA. Limited data exist about the molecular profile of index polyps as a predictive factor of metachronous lesions. Because several such polyps represent early mutational changes, they could prove useful in histological classification efforts, apart from their conventional use as carcinogenesis predictors. As expected, BRAF mutations were uncommon, although no patients with SPs were analyzed. Nevertheless, we found that 4% of patients exhibited BRAF mutation and loss of MLH1 protein expression. These polyps could reflect mixed polyps or serrated polyps mistakenly classified as adenomas. Three years may have been a too short of a period to identify molecular changes to predict recurrence (19). Juarez et al. found that polyps with KRAS mutations had a 2-fold higher risk of metachronous advanced lesions. Although we could not replicate this finding in our series, we found that KRAS mutations were useful for diagnosing HRL. This discrepancy could be because the cohort was different and that they analyzed all polyps, whereas we analyzed the most advanced polyp of each individual.
This study has several strengths. The cohort was homogenous, drawn from a CRC population screening program, and although this was a retrospective study, baseline colonoscopies were performed following an identical protocol of quality standards. Moreover, the integrity of both resections and polyp pathology analyses was ensured via second-look colonoscopies when it was deemed necessary, and the surveillance colonoscopy was performed at 3 years using the same quality standards. Finally, clinical information was obtained from computerized medical records. Thus, ours is a study with reliable results involving exhaustive analyses of clinical, endoscopic, and molecular factors associated with advanced metachronous lesions at 3 years after polypectomy.
This study also has certain limitations. Some clinical factors associated with the risk of metachronous lesions, such as diet and physical activity, were not studied, and they may be relevant. Furthermore, the CpG island methylator phenotype and microsatellite instability were not determined. Although these results would have been interesting, we instead analyzed BRAF mutations and MLH1 expression that also reflect the serrated pathway, although the correlation is not always 100%. Likewise, a greater number of cases included in the study could have helped to obtain more relevant results.
In conclusion, these results suggest that the ability to identify groups at risk of metachronous lesions remains challenging, as does the formulation of best follow-up recommendations.
Older age, female sex, smoking status, dyslipidemia, and baseline multiplicity significantly increased the risk of new advanced lesions at 3 years after polypectomy. The clinical guidelines for patient surveillance after index polypectomy should consider incorporating these clinical factors to personalize surveillance recommendations. Because no cancer was diagnosed at surveillance, we suggest that follow-up should be performed at 5 years after the procedure if the individuals do not present with risk predictive factors. Regarding MLH1 expression, we suggest examining it in all patients when conducting proximal HRL surveillance because it would be a cost-effective strategy because of the low price of the MLH1 immunohistochemistry technique.
Future studies involving larger cohorts could further confirm these findings.
CONFLICTS OF INTEREST
Guarantor of the article: Alvarez-Urturi Cristina, MD, PhD.
Specific author contributions: M.A.: conceived and designed this study. M.A., C.A.-U., L.C., and A.B.: gathered the data. A.S.-U.: contributed to the performance and evaluation of colonoscopies. G.N., D.N.-H., M.I.-C., A.D., L.F., and B.B.: processed the samples and carried out and analyzed the histopathological and molecular studies. M.A., C.A.-U., L.C., and X.B.: analyzed and interpreted the data. M.A., C.A.-U., and L.C.: drafted the article. All authors critically reviewed the draft and approved the final version of the article.
Financial support: This work was supported by grants from the Instituto de Salud Carlos III-FEDER (PI14/00441) and (PT17/0015/0011); the Singular Project AECC (PS14152544ANDR); and the Xarxa de Bancs de Tumors sponsored by Pla Director d'Oncologia de Catalunya (XBTC). AGAUR Research scholarship 2017SGR80.
Potential competing interests: None to report.
Ethics approval: The study was approved by The Hospital del Mar Clinical Research Ethics Committee (Ref. 2016S004).
Study Highlights.
WHAT IS KNOWN
✓ The risk of developing new advanced lesions is associated with different factors.
✓ Clinical follow-up recommendations are lacking in strong evidence, and surveillance strategies are based only on the size, number, and pathologic characteristics of the removed index polyps.
✓ Knowing clinical and molecular predictive factors of metachronous risk lesions would provide better risk stratification and improve surveillance programs.
WHAT IS NEW HERE
✓ Results regarding clinical, endoscopic, and molecular factors associated with risk metachronous lesions at 3 years.
✓ This study confirms that multiplicity is a strong predictor of metachronous HRLs, besides the relevance such factors as a history of smoking, dyslipidemia, older ager, female sex, and loss of MLH1 protein expression.
✓ These results expose the need to optimize current surveillance guidelines strategies.
TRANSLATIONAL IMPACT
✓ We suggest following up at 5 years if individuals do not present with clinical risk predictive factors.
✓ We propose examining patients with HRLs considering that it would be a cost-effective strategy because of low price of the MLH1 immunohistochemistry technique.
Supplementary Material
ACKNOWLEDGMENTS
We thank Xavier Duran for statistical analysis support. In addition, we are deeply grateful to the patients who participated in this investigation. We would like to thank Editage (www.editage.com) for English language editing. This study will be part of Laura Carot Bastard's doctoral thesis.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A498; http://links.lww.com/CTG/A499
Contributor Information
Laura Carot, Email: 99942@parcdesalutmar.cat.
Gemma Navarro, Email: 19526@parcdesalutmar.cat.
Dolores Naranjo-Hans, Email: 61913@parcdesalutmar.cat.
Mar Iglesias-Coma, Email: 93284@parcdesalutmar.cat.
Alba Dalmases, Email: 61499@parcdesalutmar.cat.
Lierni Fernández, Email: lierni.fdez@gmail.com.
Agustín Seoane, Email: 92847@parcdesalutmar.cat.
Andrea Buron, Email: 96036@parcdesalutmar.cat.
Beatriz Bellosillo, Email: 94161@parcdesalutmar.cat.
Xavier Bessa, Email: 93282@parcdesalutmar.cat.
REFERENCES
- 1.Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med 1992;326(10):658–62. [DOI] [PubMed] [Google Scholar]
- 2.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med 1993;329(27):1977–81. [DOI] [PubMed] [Google Scholar]
- 3.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: Review and recommendations from an expert panel. Am J Gastroenterol 2012;107(9):1315–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.East JE, Atkin WS, Bateman AC, et al. British Society of Gastroenterology position statement on serrated polyps in the colon and rectum. Gut 2017;66(7):1181–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366(8):687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arditi C, Peytremann-Bridevaux I, Burnand B, et al. Appropriateness of colonoscopy in Europe (EPAGE II) Screening for colorectal cancer. Endoscopy 2009;41:200–8. [DOI] [PubMed] [Google Scholar]
- 7.He X, Hang D, Wu K, et al. Long-term risk of colorectal cancer after removal of conventional adenomas and serrated polyps. Gastroenterology 2020;158(4):852–61.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song M, Emilsson L, Bozorg SR, et al. Articles. Risk of colorectal cancer incidence and mortality after polypectomy : A Swedish record-linkage study. Lancet Gastroenterol Hepatol 2020;1253(20):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009;136(3):832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Heijningen EB, Lansdorp-Vogelaar I, Kuipers EJ, et al. Features of adenoma and colonoscopy associated with recurrent colorectal neoplasia based on a large community-based study. Gastroenterology 2013;144(7):1410–8. [DOI] [PubMed] [Google Scholar]
- 11.Ana BM, Mercedes AM, Adán Merino L, et al. Factors related to colorectal cancer in advanced adenomas and serrated polyps. Eur J Gastroenterol Hepatol 2018;30(11):1337–43. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JC, Butterly LF, Robinson CM, et al. Risk of metachronous high-risk adenomas and large serrated polyps in individuals with serrated polyps on index colonoscopy: Data from the New Hampshire Colonoscopy Registry. Gastroenterology 2018;154(1):117–27.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonnington SN. Surveillance of colonic polyps: Are we getting it right? World J Gastroenterol 2016;22(6):1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143(3):844–57. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs ET, Ahnen DJ, Ashbeck EL, et al. Association between body mass index and colorectal neoplasia at follow-up colonoscopy: A pooling study. Am J Epidemiol 2009;169(6):657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashbeck EL, Jacobs ET, Martinez ME, et al. Components of metabolic syndrome and metachronous colorectal neoplasia. Cancer Epidemiol Biomarkers Prev 2009;18(4):1134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flood A, Mai V, Pfeiffer R, et al. Elevated serum concentrations of insulin and glucose increase risk of recurrent colorectal adenomas. Gastroenterology 2007;133(5):1423–9. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, Sun H, Yi S, et al. Molecular markers of carcinogenesis for risk stratification of individuals with colorectal polyps: A case-control study. Cancer Prev Res 2014;7(10):1023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berger AW, Raedler K, Langner C, et al. Genetic biopsy for prediction of surveillance intervals after endoscopic resection of colonic polyps: Results of the GENESIS study. United European Gastroenterol J 2018;6(2):290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juárez M, Egoavil C, Rodríguez-Soler M, et al. KRAS and BRAF somatic mutations in colonic polyps and the risk of metachronous neoplasia. PLoS One 2017;12(9):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maltzman T, Knoll K, Martinez ME, et al. Ki-ras proto-oncogene mutations in sporadic colorectal adenomas: Relationship to histologic and clinical characteristics. Gastroenterology 2001;121(2):302–9. [DOI] [PubMed] [Google Scholar]
- 22.Lochhead P, Chan AT, Nishihara R, et al. Etiologic field effect: Reappraisal of the field effect concept in cancer predisposition and progression. Mod Pathol 2015;28(1):14–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valori R, Rey J-F, Atkin W, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition—Quality assurance in endoscopy in colorectal cancer screening and diagnosis. Endoscopy 2012;44(S 03):SE88–105. [DOI] [PubMed] [Google Scholar]
- 24.Carvajal-Carmona LG, Cazier JB, Jones AM, et al. Fine-mapping of colorectal cancer susceptibility loci at 8q23.3, 16q22.1 and 19q13.11: Refinement of association signals and use of in silico analysis to suggest functional variation and unexpected candidate target genes. Hum Mol Genet 2011;20(14):2879–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults. JAMA 2014;311(5):507. [DOI] [PubMed] [Google Scholar]
- 26.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech 2009;2(5–6):231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopanos C, Tsiolkas V, Kouris A, et al. VarSome: The human genomic variant search engine. Bioinformatics 2018;35(11):1978–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okanoue T, Tatsumi Y, Hattori T, et al. Expression of cytokeratins 7 and 20 in serrated adenoma and related diseases. Dig Dis Sci 2005;50(9):1741–6. [DOI] [PubMed] [Google Scholar]
- 29.Winn B, Tavares R, Matoso A, et al. Expression of the intestinal biomarkers guanylyl cyclase C and CDX2 in poorly differentiated colorectal carcinomas. Hum Pathol 2010;41(1):123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosty C, Clouston AD, Klein K, et al. A clinicopathological and molecular analysis of 200 traditional serrated adenomas. Mod Pathol 2014;28(3):414–27. [DOI] [PubMed] [Google Scholar]
- 31.Bonithon-Kopp C, Piard F, Fenger C, et al. Colorectal adenoma characteristics as predictors of recurrence. Dis Colon Rectum 2004;47(3):323–33. [DOI] [PubMed] [Google Scholar]
- 32.Leufkens A, van Oijen M, Vleggaar F, et al. Factors influencing the miss rate of polyps in a back-to-back colonoscopy study. Endoscopy 2012;44(05):470–5. [DOI] [PubMed] [Google Scholar]
- 33.Gkolfakis P, Tziatzios G, Facciorusso A, et al. Meta-analysis indicates that add-on devices and new endoscopes reduce colonoscopy adenoma miss rate. Eur J Gastroenterol Hepatol 2018;30(12):1482–90. [DOI] [PubMed] [Google Scholar]
- 34.Zhao S, Wang S, Pan P, et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: A systematic review and meta-analysis. Gastroenterology 2019;156(6):1661–74.e11. [DOI] [PubMed] [Google Scholar]
- 35.Ghazi S, Lindforss U, Lindberg G, et al. Analysis of colorectal cancer morphology in relation to sex, age, location, and family history. J Gastroenterol 2012;47(6):619–34. [DOI] [PubMed] [Google Scholar]
- 36.Hirai HW, Ching JYL, Wu JCY, et al. Risk factors for advanced colorectal neoplasms in the proximal colon in 6218 subjects undergoing complete colonoscopy. J Gastroenterol Hepatol 2019;34(1):113–9. [DOI] [PubMed] [Google Scholar]
- 37.McCashland TM, Brand R, Lyden E, et al. Gender differences in colorectal polyps and tumors. Am J Gastroenterol 2004;96(3):882–6. [DOI] [PubMed] [Google Scholar]
- 38.Slattery ML, Potter JD, Curtin K, et al. Estrogens reduce and withdrawal of estrogens increase risk of microsatellite instability-positive colon cancer. Cancer Res 2001;61(1):126–30. [PubMed] [Google Scholar]
- 39.Trayhurn P, Beattie JH. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc Nutr Soc 2001;60(3):329–39. [DOI] [PubMed] [Google Scholar]
- 40.Tsoi KKF, Pau CYY, Wu WKK, et al. Cigarette smoking and the risk of colorectal cancer: A meta-analysis of prospective cohort studies. Clin Gastroenterol Hepatol 2009;7(6):682–8.e5. [DOI] [PubMed] [Google Scholar]
- 41.Jung YS, Kim NH, Lee MY, et al. Effect of cotinine-verified change in smoking status on risk of metachronous colorectal neoplasia after polypectomy. Clin Gastroenterol Hepatol 2020;18(1):163–70. [DOI] [PubMed] [Google Scholar]
- 42.Patel A, Williams N, Parsons N, et al. Risk factors for metachronous adenoma in the residual colon of patients undergoing curative surgery for colorectal cancer. Int J Colorectal Dis 2017;32(11):1609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lieberman DA, Weiss DG, Harford WV, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology 2007;133(4):1077–85. [DOI] [PubMed] [Google Scholar]
- 44.Nusko G, Mansmann U, Kirchner T, et al. Risk related surveillance following colorectal polypectomy. Gut 2002;51(3):424–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martínez ME, Sampliner R, Marshall JR, et al. Adenoma characteristics as risk factors for recurrence of advanced adenomas. Gastroenterology 2001;120(5):1077–83. [DOI] [PubMed] [Google Scholar]
- 46.Rex D, Cutler C, Lemmel G, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology 1997;112(1):24–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.