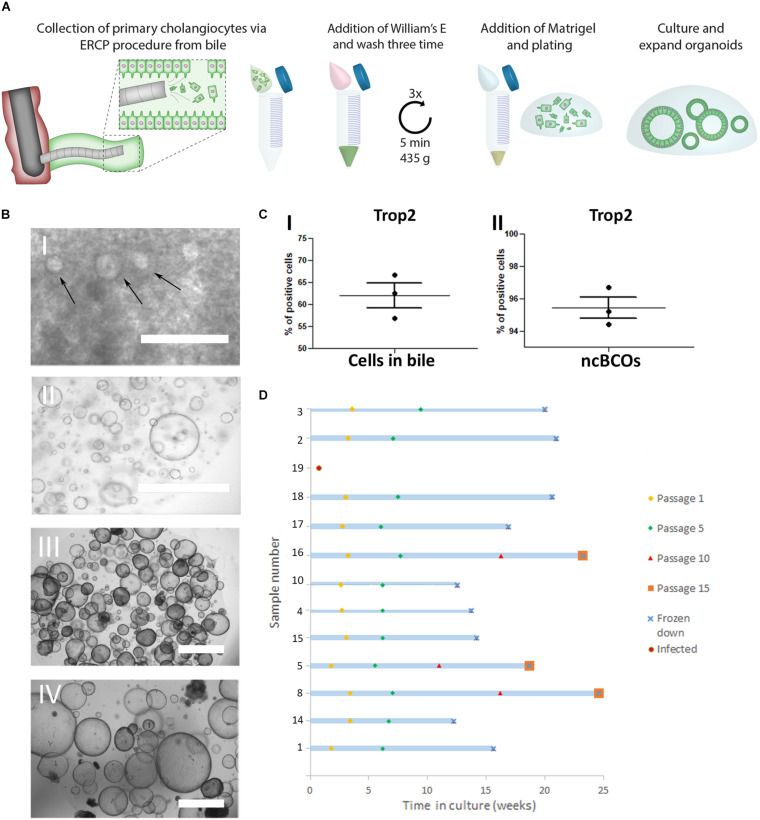
FIGURE 1.
Creation and culturing of bile cholangiocyte organoids in non-canonical Wnt stimulating conditions (ncBCOs). (A) Schematic representation of initiation of ncBCOs from endoscopic retrograde cholangiopancreatography (ERCP) collected bile. First bile is collected via an ERCP-procedure (1 ml) Organoid-initiating cells (viable cholangiocytes) are present in bile and bile should be quickly transferred on ice for processing. Next the bile was diluted and washed a total of three times with William’s-E medium, to remove all bile from the cells. If a lot of debris is present, additional cells could be passed through a 70 μm cell strained. Subsequently, a hydrogel (Matrigel, Corning or base membrane extract, Cultrex) is added and the cells are plated out in 25 μl of hydrogel droplets and cholangiocytes are expended as organoids. (B) (i) A representative image of ncBCOs at passage 0 (P0) after 5 days in culture. (ii) P5 ncBCOs showing mostly cyst-like organoids. (iii) A representative of an extrahepatic cholangiocyte organoid in non-canonical Wnt stimulating conditions (ncECO) P3. (iv) ncECO passage P5. All scale bar indicates 1,000 μm. (C) (i) Trop2 flow cytometry of all cells counted as an event in bile, indicating that Trop2pos cells are dominantly present in bile, in (ii) the mean percentage of Trop2poscells in ncBCOs are displayed. (D) Number of passages of the 13 ncBCOs cultured before cryopreservation and storage.