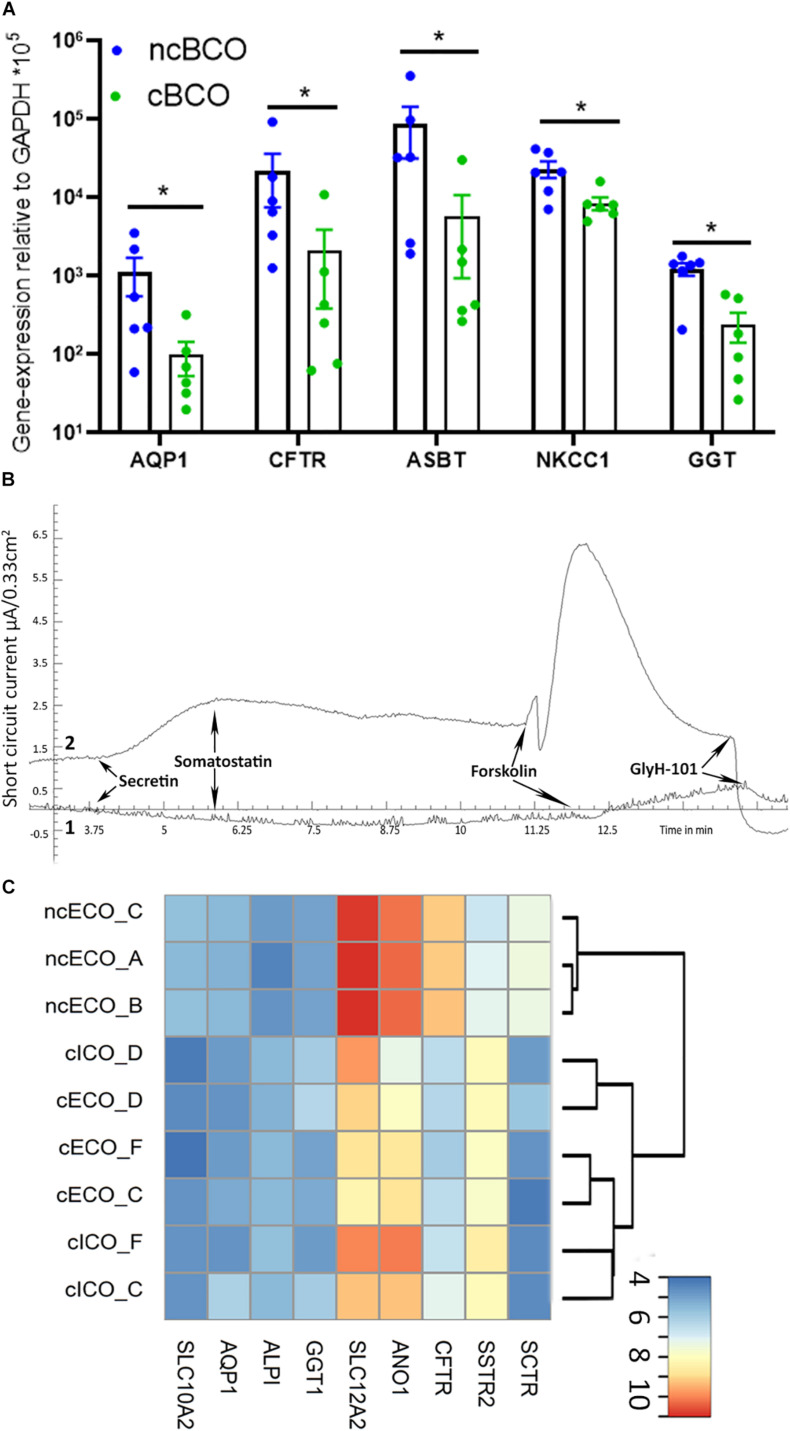
FIGURE 4.
ncBCOs retain secretin receptor responsiveness and increased ion channel activity in vitro compared to cBCOs. (A) qRT-PCR of ncBCOs and cBCOs (both, n = 6), for cholangiocyte specific channels and transporters. Showing a significant upregulation (p < 0.05, as indicated by *) for AQP1, CFTR, ASBT, NKCC-1, and GGT in ncBCOs compared to cBCOs. Error bars are displayed as SEM. (B) Representative ion-channel functionality of 2D-grown bile-cholangiocyte organoids in non-canonical Wnt-stimulated conditions (ncBCOs, line 2) and bile cholangiocyte organoids in canonical-Wnt-stimulated organoids (cBCOs, line 1) in an Ussing chamber, stimulation with cAMP-activator (forskolin), resulted in an increase in short circuit current; however, secretin stimulation (to the basolateral side) only gave a response in the ncBCOs. In similar fashion, somatostatin (basolateral addition) only give a response in ncBCOs and not in cBCOs, while CFTR inhibition via GlyH-101 (luminal addition), resulted in an inhibition of the channel in both organoid-types, indicating the presence of functional CFTR channels in both organoids, but only somatostatin and secretin receptors are functional in ncECOs. Moreover, it seems that CFTR-function is higher in organoids in non-canonical Wnt stimulating conditions compared to organoids in canonical-Wnt-stimulating conditions. (C) Heatmap and clustering based expression of cholangiocyte-related gene expression as analyzed by gene array for functional enzymes (ALPI and GGT1), channels (SLC12A2—also known as NKCC1-, SLC10A2—also known as ASBT-, AQP1, ANO1, and CFTR) and receptors (SCTR and SSTR) between cICOs (n = 3), cECOs (n = 3), and ncECO (n = 3). Color key represents the log2 transformed signal intensities after variance stabilizing normalization.