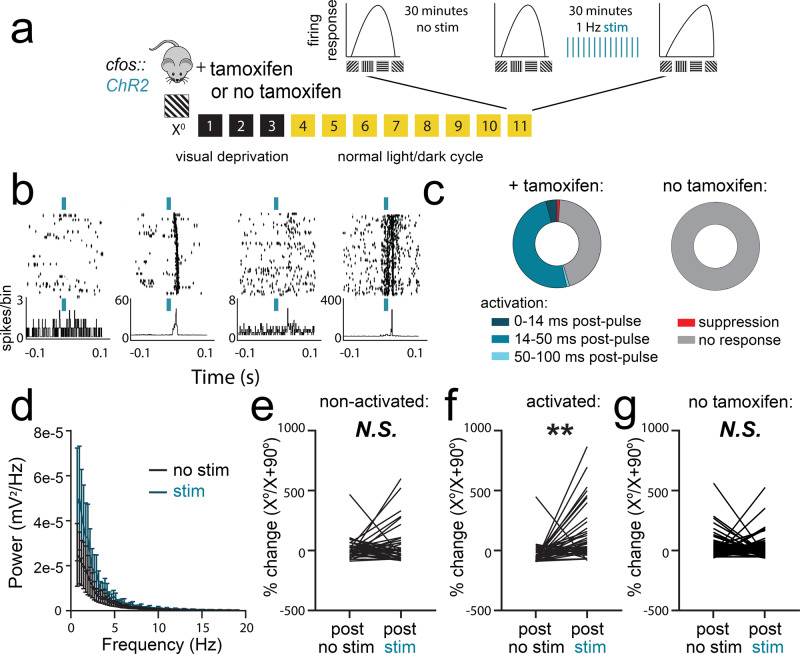
Fig. 5. Offline reactivation of orientation-selective TRAPed V1 neurons alters orientation representations in V1.
a cfos::ChR2 mice were presented with an oriented grating (X°) for TRAP. A second cohort was treated identically, without tamoxifen administration to induce recombination (no tamoxifen). Eleven days later, orientation tuning was measured repeatedly for V1 neurons recorded from anesthetized mice: at baseline, after a 20–30-min period without optogenetic stimulation, and after a 20–30-min period with 1 Hz light delivery. b Representative rasters and perievent histograms for four simultaneously recorded neurons, showing diverse firing responses during optogenetic stimulation of ChR2-expressing neurons. c The majority of stably recorded V1 neurons were reliably activated following light pulses, with variable lag times. A small proportion were inhibited by light delivery, and the remaining neurons were not affected (n = 96 neurons from 6 mice, total). Neurons recorded during rhythmic light delivery in control (no tamoxifen) mice showed no responses to light pulses (n = 79 neurons from 5 mice, total). d Power spectra for V1 LFPs showed no significant effect on ongoing rhythmic activity (N.S., K–S test, n = 5 mice). Values indicate mean ± SEM. e, f After optogenetic stimulation, neurons that were not activated following light pulses showed no change in orientation preference (N.S., nested t test, n = 37 neurons from 5 mice). In contrast, activated neurons showed increased firing rate responses for gratings of the same orientation (X°) used for TRAP. (**p = 0.004 [t = 3.93, DF = 8], nested t test, n = 40 neurons from 5 mice). g Neurons in control (no tamoxifen) mice showed no consistent orientation preference changes following rhythmic light delivery (N.S., nested t test, n = 79 neurons from 5 mice).