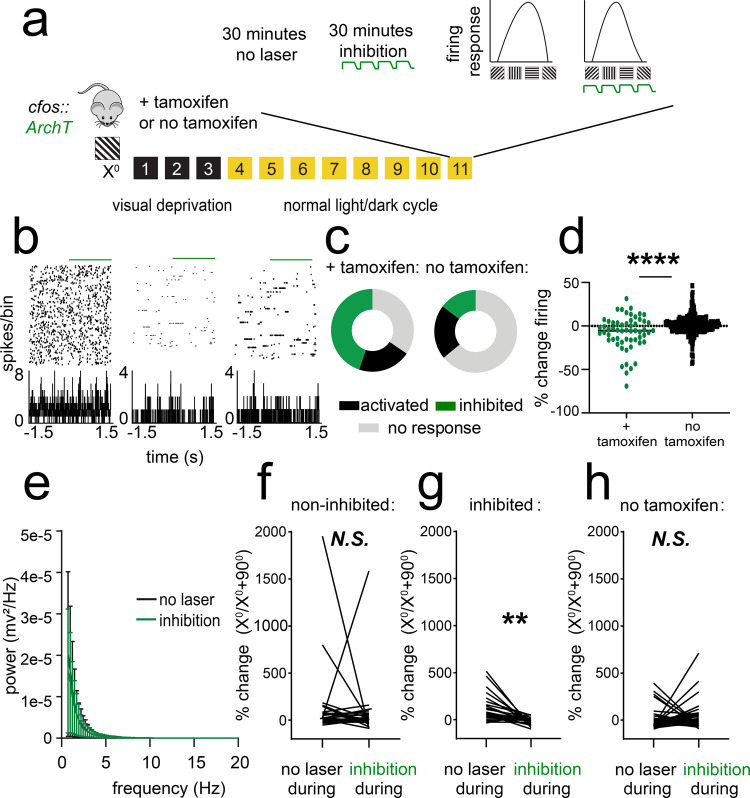
Fig. 6. Optogenetic inhibition of orientation-selective TRAPed V1 ensembles alters orientation preference in surrounding V1 neurons.
a cfos::ArchT mice were presented with an oriented grating (X°) for TRAP. Eleven days later, V1 neurons were recorded from anesthetized mice across 30 min of optogenetic inhibition and 30 min without inhibition. Afterward, orientation preference was assessed at baseline, during a control period without optogenetic inhibition (no laser), and during a period with inhibition delivered at the same time as visual stimuli. A second cohort was treated identically, without tamoxifen administration to induce recombination (no tamoxifen). b Representative rasters and perievent histograms for 3 simultaneously recorded neurons from an Arch-expressing mouse, showing diverse firing responses during periodic optogenetic inhibition. c Distributions of stably recorded V1 neurons, which were inhibited (with >5% decrease in firing rate), activated (with >5% increase in firing rate), or unaffected by light delivery (n = 58 neurons from 5 tamoxifen treated mice, n = 278 neurons from 4 no tamoxifen control mice). d Firing rate changes with light delivery were significantly greater for Arch-expressing mice than for no tamoxifen control mice (****p = 0.0001, Mann–Whitney test). e Power spectra for V1 LFPs showed no significant change in rhythmic activity during periods of inhibition (N.S., K–S test, n = 5 mice). Values indicate mean ± SEM. f, g Neurons recorded from Arch-expressing mice that showed no decrease in firing rate during light delivery showed no change in orientation preference when light was delivered to V1 during presentation of visual stimuli (N.S., nested t test, n = 32 neurons from 5 mice). In contrast, neurons that were inhibited showed a reduced preference for gratings of the same orientation (X°) used for TRAP (**p = 0.007 [t = 3.65, DF = 8, nested t test, n = 26 neurons from 5 mice). h Neurons recorded from no tamoxifen control mice showed no consistent change in orientation preference when light was delivered to V1 during presentation of visual stimuli (N.S., nested t test, n = 52 neurons from 3 mice).