Fig. 5. The link between c-di-AMP and (p)ppGpp signaling in B. subtilis.
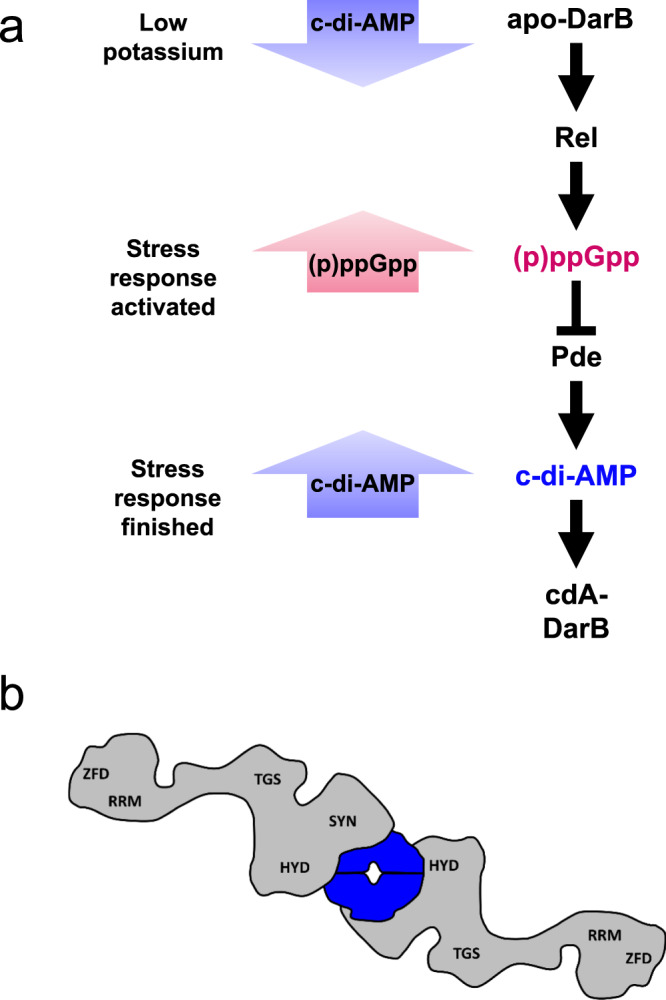
a The model depicts the bidirectional and dynamic process of the cellular response to potassium limitation. When potassium becomes limiting, the diadenylate cyclases respond and produce less c-di-AMP and c-di-AMP receptor proteins are present in the apo-form. One apo-DarB dimer binds to Rel and by this disrupts the Rel dimer. This leads to formation of the DarB–Rel complex as suggested by the presented data. One DarB dimer is bound by two Rel monomers, one on each side. The interaction occurs via the HYD-SYN domains of Rel. Interaction of DarB with Rel leads to stimulation of (p)ppGpp synthesis. (p)ppGpp accumulation induces the stringent response and inhibits the c-di-AMP-degrading phosphodiesterases GdpP and PgpH. This leads to increasing intracellular c-di-AMP amounts. DarB can then bind c-di-AMP and is thus no longer able to interact with Rel. b The model shows the DarB-Rel complex as suggested by our data. One DarB dimer is bound by two Rel monomers, one on each side. The interaction occurs via the HYD-SYN domains of Rel. DarB, blue; Rel, gray. cdA, c-di-AMP; HYD, hydrolase domain; SYN, synthetase domain; TGS, TGS domain (for: ThrRS, GTPase, and SpoT); ZFD, Zinc finger domain; RRM, RNA Recognition Motif.
