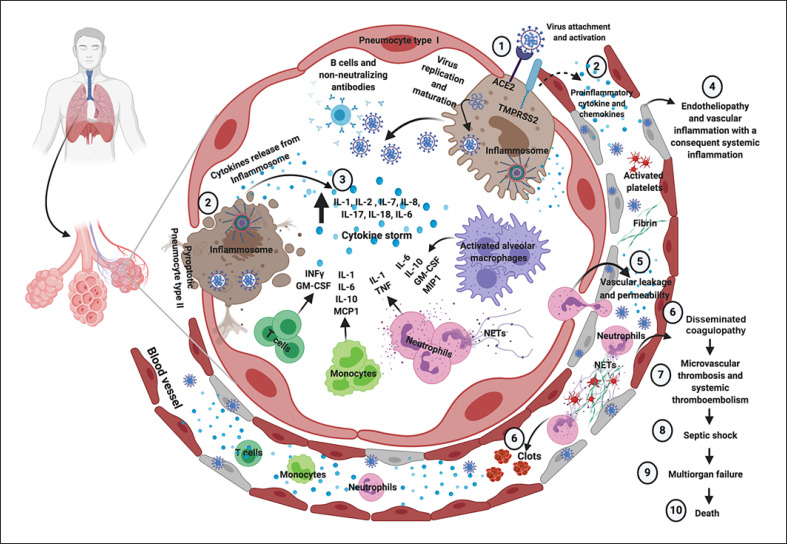
Fig. 1.
Immune, inflammatory, and thrombotic response to SARS-CoV-2 infection in case of severe COVID-19. SARS-CoV-2 enters into cells expressing the surface ACE2 receptors and TMPRSS2 (1). The replication and release of SARS-CoV-2 cause pyroptosis of host cells and release of proinflammatory cytokines by inflammasomes (mainly IL-1, IL-8, and IL-18) and cell debris that activate alveolar macrophages, which in turn further release proinflammatory cytokines (mainly IL-10, GM-CSF, and MIP1) and chemokines (2). These proteins attract other innate and adaptive immune cells in the lungs, damaging the lung infrastructure and with the addition of increasing release of IFN-γ by T cell promoting a proinflammatory feedback loop and cytokine storm (3). Moreover, production of non-neutralizing antibodies by B cells may enhance SARS-CoV-2 infection, further exacerbating organ damage. Concomitantly, the damage of endothelial tissue directly caused by SARS-CoV-2 entry and the local inflammation induce endotheliopathy characterized by injured endothelial tissue (4) with consequent vascular leaking (5). Neutrophil extracellular traps induce the aggregation of platelets and fibrin deposition, leading to blood clots formation and promoting disseminated coagulopathy (6). This mechanism finally results in microvascular thrombosis and systemic thromboembolism (7). As a consequence, septic shock and multiorgan failure may develop and represent potential major death determinants in COVID-19. Figure created with www.biorender.com. ACE2, angiotensin-converting enzyme 2; COVID-19, coronavirus disease 2019; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon gamma; IL, interleukin; MCP1, monocyte chemoattractant protein 1; MIP1, macrophage inflammatory protein 1; NETs, neutrophil extracellular traps; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMPRSS2, transmembrane protease serine 2; TNF, tumor necrosis factor.