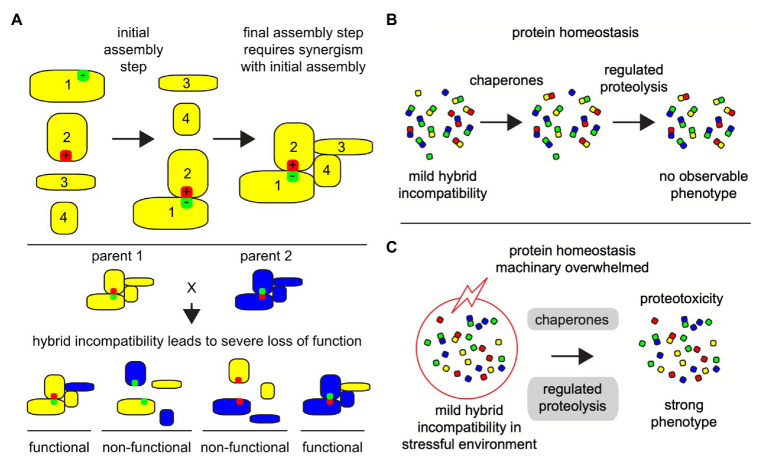
Figure 2.
Co-evolving protein-protein interactions depend on the phenotypic traits and stressful environmental and cellular conditions can reveal hybrid incompatibility phenotypes. Illustrated examples of protein micro-environment in a protein-protein complex structural and functional integrity. (A) Large multi-subunit protein complexes are assembled in a step-by-step manner, where hybrid incompatibility may lead to the loss of the entire functional complex, especially if one of the evolved binding partners is necessary for an early assembly step. In this illustration protein 1 and protein 2 must assemble first in order to promote a stable interaction with proteins 3 and 4. If mutation-suppression occurs in divergent organisms, such as the reverse of positively (red) and negatively (green) charged amino acid residues as illustrated here, then a severe disruption in the protein complex assembly can occur (bottom). (B) Under congenial environments, when cells are challenged by mild forms of intrinsic protein-protein incompatibilities, the cellular homeostasis machinery, including chaperones and regulated proteolysis, protects the cells and promotes the formation and selection of functional protein complexes. (C) Even under weak stressful environments (red circle with lightning), mild protein-protein incompatibilities can accentuate to higher levels of unfolded and mis-assembled proteins, leading many cellular complexes to fail and overwhelm the protein homeostasis machinery (gray) causing a collapse in cellular protein homeostasis and proteotoxic stress.