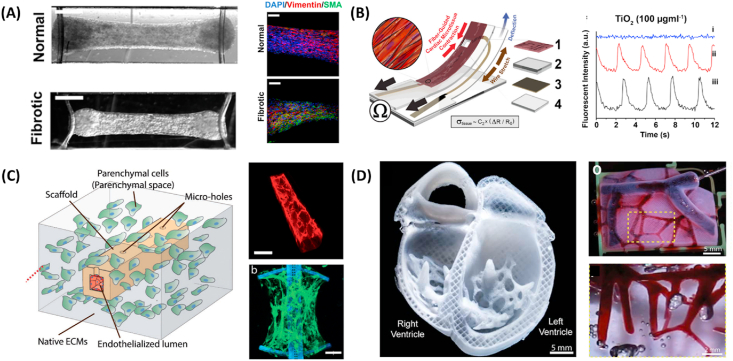
Fig. 4.
Representative heart-on-a-chip systems for understanding nanoparticle induced toxicity. (A) Matured cardiac tissue for investigation of cardiac fibrosis (left). Fibrotic tissue exhibited higher expression of vimentin (green) as illustrated in the immunostaining image of cardiac and fibrotic tissue (right). Reproduced with permission [205]. Copyright 2019, American Chemical Society. (B) Fibrin-coated cardiac microphysiological device for contractility assessment. Exposure to TiO2 nanoparticles decreased contractile function of cardiac tissue through disruption of sarcomeric structure. Reproduced with permission [208]. Copyright 2018, Springer Nature. (C) Confocal image of perfusable vascularized scaffold that supports the self-assembly of cardiac tissue (red: CD31, green: sarcomeric α-actinin). This system allows the investigation of complex interaction between endothelial cells and nanoparticles, and their subsequent cardiac toxicity under electrical stimulation in 96-well format. Reproduced with permission [131]. Copyright 2017, Advanced Functional Materials. (D) 3D bioprinted neonatal-scale human heart with multi-scale vasculature composed of collagen material (left). Perfusion of glycerol (red) through coronary artery also permits the perfusion down to vessels ~100 μm in diameter (right). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Reproduced with permission [208]. Copyright 2018, Springer Nature.