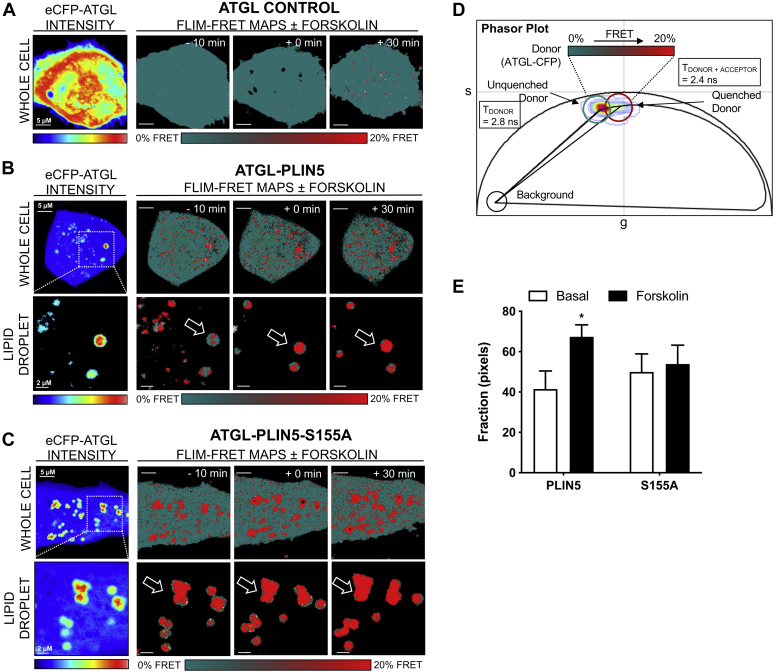
Fig. 3.
Phasor-based FLIM-FRET reveals requirement of PLIN5 phosphorylation at S155 to mediate interaction with ATGL. A: Intensity images acquired in the donor (CFP) (A: left) channel of live HeLa cells expressing ATGL-CFP in the absence (denoted ATGL control) of PLIN5-YFP or PLIN5 S155A-YFP before and after stimulation with 20 μM forskolin. Fluorescence lifetime images acquired in the donor (CFP) (A: right) channel of the different live HeLa cells pseudo-colored to report no FRET (teal pixels, 2.8 nanoseconds [ns]) versus FRET (red pixels, 2.4 ns). B, C: FRET analysis of FLIM images with an intensity threshold applied to select only the lipid droplets in HeLa expressing ATGL-CFP in the presence of PLIN5-YFP (B) versus S155A-YFP (C) before and after stimulation with 20 μM forskolin. D: Superimposition of the combined phasor distribution of ATGL-CFP in the absence (unquenched donor control, teal cursor) versus presence of PLIN5-YFP or S155A-YFP (FRET experiments, red cursor) with a theoretical FRET trajectory superimposed. The FRET efficiency of ATGL-PLIN5 interaction is 20%. E: Quantitation of the number of pixels undergoing FRET in FLIM images of HeLa expressing ATGL-CFP in the absence versus presence of PLIN5-YFP or PLIN5 S155A-YFP before and after 20 μM forskolin administration. Data presented as mean ± SEM for n = 11–13 cells from three independent experiments. For panel (E), two-way ANOVA with Bonferroni post hoc analysis was performed. ∗P < 0.05 basal versus forskolin.