Fig. 6.
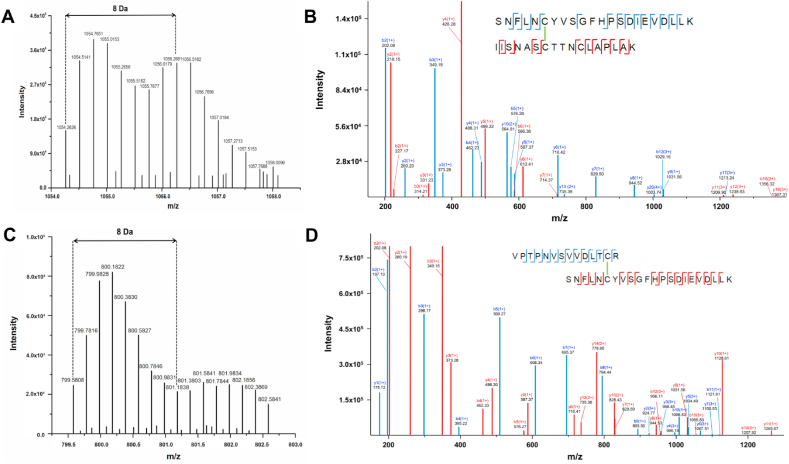
New inter-protein disulfides are formed by oxidation of disulfide bonds and subsequent reaction with protein thiols. Panel A: MS spectrum of the quadruply charged Cys-Cys cross-linked peptide with m/z 1054.26 displaying a +8 Da shift following trypsin digestion in H218O versus H216O (1:1 ratio). Panel B: Assignment of a MS/MS spectrum of the cross-linked peptide (SNFLNCYVSGFHPSDIEVDLLK) (IISNASCTTNCLAPLAK) with the first peptide (peptide A) containing Cys25 from B2M, and the second peptide (peptide B) containing Cys149 from GAPDH. Panel C: MS spectrum of the quintuply charged Cys-Cys cross-linked peptide at m/z 799.58 displaying a +8 Da shift. Panel D: Assignment of a MS/MS spectrum of the cross-linked peptide (VPTPNVSVVDLTCR) (SNFLNCYVSGFHPSDIEVDLLK) with the first peptide (peptide A) containing Cys244 from GAPDH and the second peptide (peptide B) containing Cys25 from B2M. Blue b and y ions correspond to the peptide A, while red b and y fragments correspond to the peptide B. Spectra were obtained from the software Data Analysis, with the fragment ions annotated manually. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)