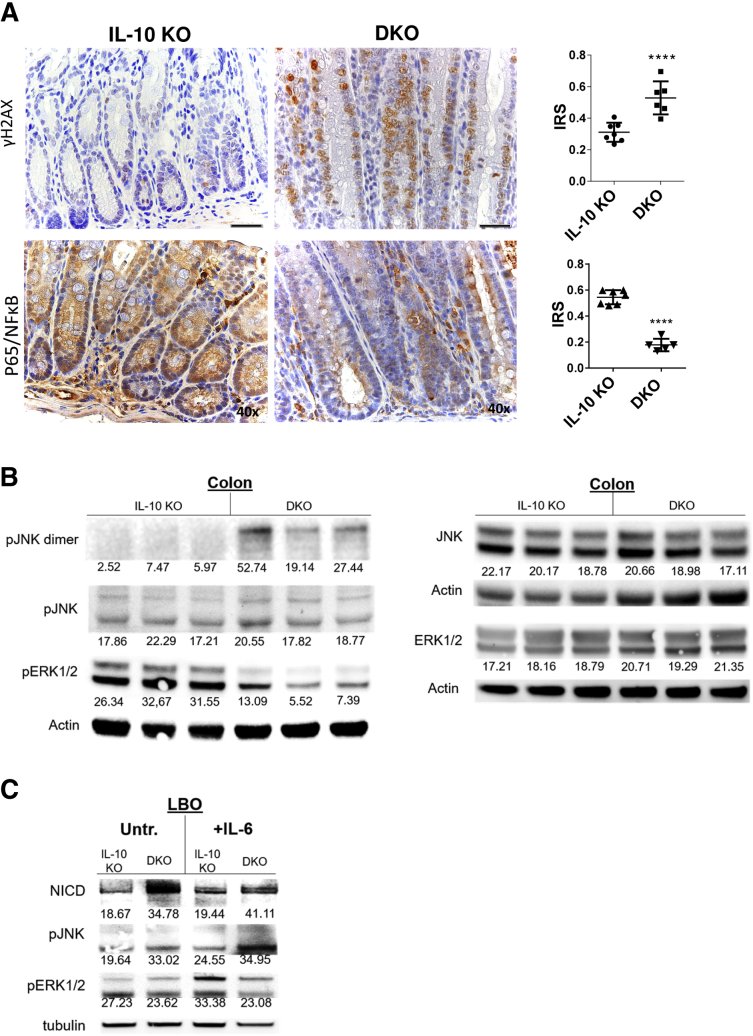
Figure 5.
PAK1 deficiency exacerbates oxidative stress–related DNA damage and stress kinases, and drives Notch1 activation. (A) Immunohistochemistry (IHC) of γH2AX marking double-strand breaks. PAK1 deletion resulted in increased double-strand breaks. IHC of p65 indicating NF-κB activation and corresponding IRS. PAK1 deletion attenuated NF-κB signaling. (B) Western blot of colon samples from IL10 KO and DKO mice. In DKO mice, pERK signaling was abrogated, while blotting for pJNK showed increased dimerization, indicating pJNK activation. (C) Western blot of LBOs, which were treated with IL6 (10 ng/mL). Treatment resulted in activation of Notch1 and JNK in DKO, while ERK/AKT activation was impeded upon PAK1 loss. AKT, protein kinase B; γH2AX, phosphorylated H2A histone family member X; IRS, immune-reactivity score; pERK, phosphorylated extracellular signal-regulated kinase1/2; pJNK, phosphorylated c-Jun N-terminal kinase.