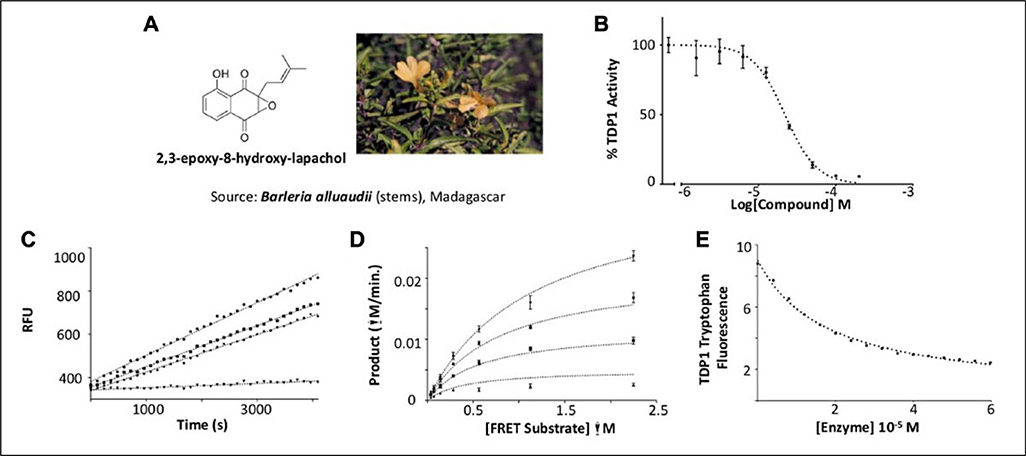
Figure 4.
2,3-epoxy-8-hydroxy-lapachol as an inhibitor of human tyrosyl-DNA phosphodiesterase 1 (TDP1). (A) 2,3-epoxy-8-hydroxy-lapachol was isolated from the stems of Barleria alluaudii. (B) Dose-response analysis yields an IC50 of 21.9 ± 0.32 μM. (C) Reversibility studies of 2,3-epoxy-8-hydroxy-lapachol (◆) and aurintricarboxylic acid (■) show recovery of 90% of the uninhibited control rate (●) after preincubation with compound for 1 h. Halenoquinone shows no such recovery of activity (▼), indicating irreversible inhibition. (D) A series of substrate saturation curves carried out in the presence of 0 μM (◆), 6.6 μM (●), 20 μM (■), and 60 μM (▲) 2,3-epoxy-8-hydroxy-lapachol produced a pattern of inhibition best described by model of a mixed-type noncompetitive mechanism. (E) Binding of 2,3-epoxy-8-hydroxy-lapachol to the TDP1 apoprotein was observed by monitoring the decrease in intrinsic tryptophan fluorescence under increasing concentrations of compound (●). The equilibrium dissociation constant for the interaction (Kd) was estimated to be 19.1 ± 0.7 μM.