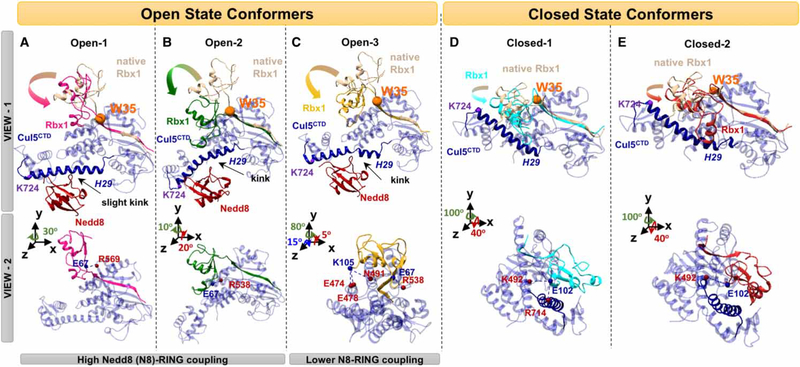
Figure 2. RBX RING domain rotations.
(A–C) Nedd8 (red) conjugated CRLCTD (open state) conformers that have three distinct Rbx1 RING domain rotations, and (D and E) unmodified CRLCTD (closed state) conformers are shown in two different views. The H29 of Cul (blue) are highlighted by making the rest of Cul elements transparent. Distinct Rbx1 conformations are shown with different colors (pink, green, and yellow for open; cyan and red for closed). Rotations from native — crystal structure — Rbx1 (beige) are highlighted with arrows. The Rbx1 W35 residue shown with an orange sphere has a pivotal role in RING domain rotations after (A–C) and before (D and E) Cul neddylation. Nedd8-binding site on CulCTD, K724, is shown with a purple sphere in (D and E). In view 2, the H-bonds between Cul residues (red spheres) and Rbx1 (blue spheres) stabilizing the Rbx1 RING domain conformation are shown for both states.