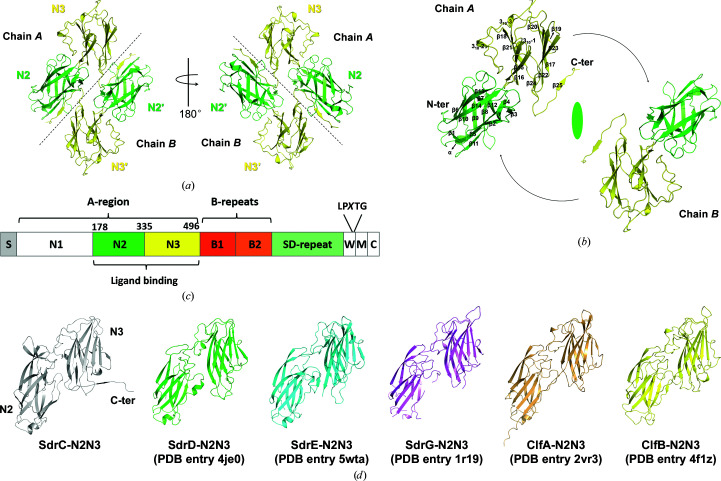
Figure 1.
Overall structure of SdrC-N2N3. (a) Crystal structure of SdrC. The N2 and N3 subdomains are colored green and yellow, respectively. Black dotted lines roughly separate the two chains. (b) Relationship of the two chains in the dimer. Secondary-structure elements are indicated on chain A. The dyad axis is shown as an ellipsoid and black arrows show the rotation between the two monomers. (c) Structural organization of SdrC. S, signal sequence, The A-region includes N1–N3. The ligand-binding region, N2N3, is located within the A-region. The B-repeats include B1 and B2. SD-repeat, Ser-Asp dipeptide repeats. W, short wall-spanning region. M, membrane-spanning segment. C, intracellular region. The LPXTG motif is located between the W and M domains. (d) Structural comparison of SdrC with other Clf–Sdr family proteins. One copy (chain A) was extracted from the apo structures of SdrC-N2N3 (gray), SdrD-N2N3 (green), SdrE-N2N3 (cyan) and SdrG-N2N3 (violet) and the structures of the ClfA–Fg (light orange) and ClfB–keratin (yellow) complexes. The overall structure of the six proteins was composed of two Ig-like subdomains with a similar arrangement.