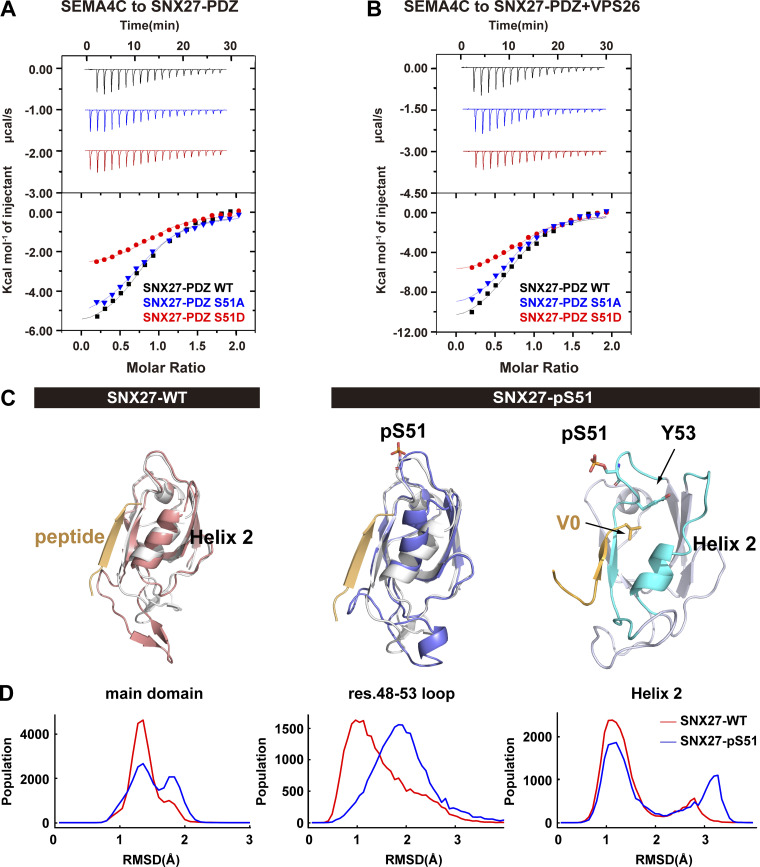
Figure 5.
Phosphorylation of Ser51 reduces SNX27 binding to PDZbm-containing cargoes via altering the conformation of the cargo-binding pocket. (A) Isothermal titration calorimetry-binding curves for SEMA4C (aa 686–833) titrated into SNX27-PDZ WT, Ser51A, or Ser51D at 25°C. Top and bottom panels show raw and integrated heat from injections, respectively. The black curve in the bottom panel represents a fit of the integrated data to a single-site binding model. (B) ITC-binding curves for SEMA4C (aa 686–833) titrated into SNX27-PDZ WT, Ser51A, or Ser51D supplemented with equimolar VPS26 at 25°C. Top and bottom panels show raw and integrated heat from injections, respectively. The black curve in the bottom panel represents a fit of the integrated data to a single-site binding model. (C) MD-simulated conformations of SNX27 PDZ WT (red) and pSer51(blue), and comparison with the crystal structure (white, PDB accession no. 5ELQ). DGKζ peptide is shown in orange. (D) Distribution of root-mean square deviation (RMSD) between MD-simulated conformations and the crystal structure of SNX27 PDZ (PDB accession no. 5ELQ). Main domain: entire PDZ domain excluding residue (res.) 61–77 loop; residue 48–53 loop; helix 2.