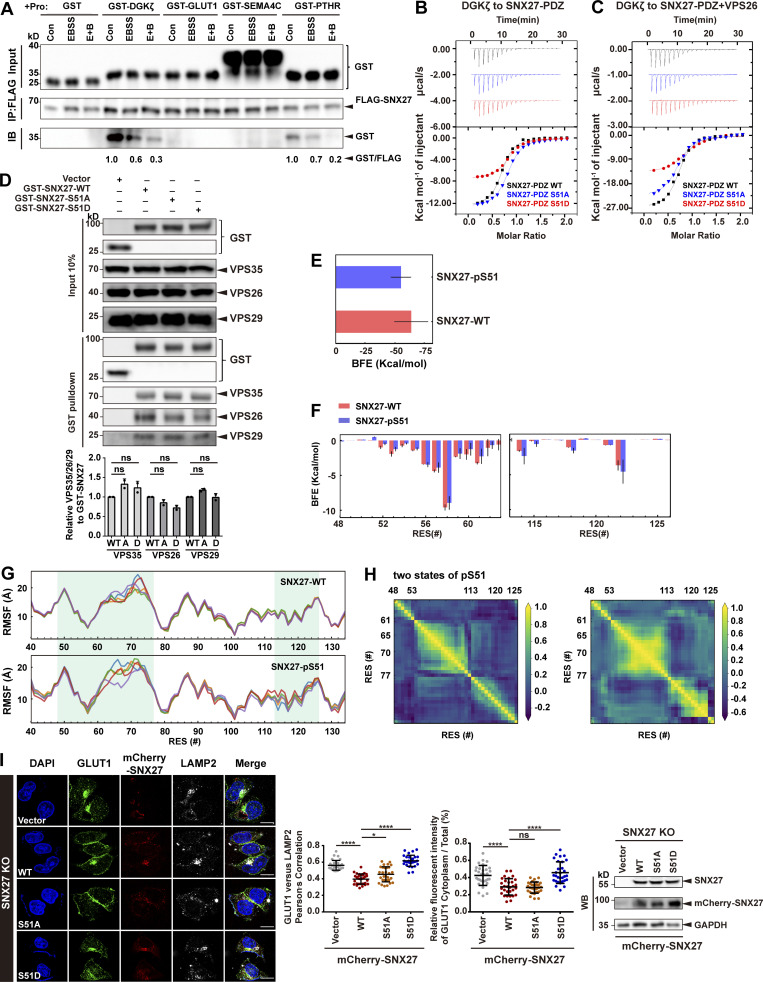
Figure S4.
Phosphorylation of Ser51 reduces SNX27 binding to its cargoes. (A) Starvation decreases the interaction between SNX27 and PDZbm-containing proteins. HEK293T cells were transfected with FLAG-tagged SNX27 for 24 h and starved or not for 4 h. The whole-cell lysates were immunoprecipitated with anti-FLAG beads, followed by incubation with four purified GST-tagged PDZbms (DGKζ, GLUT1, SEMA4C, and PTHR) or GST. The bound samples were then washed and analyzed by immunoblotting. Representative of three independent iterations. The bands of GST-tagged DGKζ and PTHR were quantified by ImageJ, normalized to the level of the respective control group, and labeled below the blots. (B) ITC-binding curves for DGKζ peptide (REDQETAV) titrated into SNX27-PDZ WT, Ser51A, or Ser51D at 25°C. Top and bottom panels show raw and integrated heat from injections, respectively. The black curve in the bottom panel represents a fit of the integrated data to a single-site binding model. (C) ITC-binding curves for DGKζ peptide (REDQETAV) titrated into SNX27-PDZ WT, Ser51A, or Ser51D supplemented with equimolar VPS26 at 25°C. Top and bottom panels show raw and integrated heat from injections, respectively. The black curve in the bottom panel represents a fit of the integrated data to a single-site binding model. (D) Pull-down assay to assess binding of GST-SNX27 and endogenous VPS35/26/29. HEK293T cells were transfected with GST-tagged SNX27-WT, Ser51A, or Ser51D for 24 h. Control cells (vector) were transfected with empty vector. One tenth of the cell lysate was prepared as input, and the rest was used for pull-down with GST resin, followed by immunoblotting using antibodies against GST-SNX27, VPS35, VPS26, and VPS29. Representative images from one of two independent experiments are shown. Column chart represents the relative expression of VPS35/26/29 to GST-SNX27-WT, Ser51A, or Ser51D. Band intensities were quantified with ImageJ and normalized to their respective WT group (n = 2). Data are presented as means ± SD, and one-way ANOVA by Tukey’s HSD test or t test was used for data analysis. (E) Comparison of binding free energy (BFE) between DGKζ peptide to SNX27 PDZ WT and pSer51. (F) Per-residue free energy decomposition for sequences around the DGKζ-binding regions. (G) Residue fluctuation, measured by root mean square fluctuation (RMSF) of the entire PDZ domain in the WT and pSer51 systems. (H) Dynamic cross-correlation maps for two representative conformations of pSer51, shown in Fig. 5 B. Correlated (positive values) and anticorrelated (negative values) motions varied between residue (RES) 48-53 loop, residue 61–77 loop, or helix 2 (residues 113–126) in the two conformations. (I) Confocal imaging for GLUT1 (green) and LAMP2 (gray) immunofluorescence staining in HeLa cells. SNX27-KO cells were transfected with mCherry-tagged SNX27 WT, Ser51A or Ser51D, or empty vector, for 24 h. Representative images from two independent experiments are shown. Scale bar: 10 µm. Pearson’s correlation coefficients for GLUT1 and LAMP2. Relative fluorescent intensity of GLUT1 in cytoplasm/total ratio in each cell. Results for individual cells are plotted, along with the mean and SD for each group (n = 30); P values were calculated using one-way ANOVA by Tukey’s HSD test or t test. ****, P < 0.0001; *, P < 0.05. Knockdown and transfection efficiency were determined by immunoblotting with antibodies as indicated (right). HSD, honestly significant difference; ns, not significant.