Figure 6.
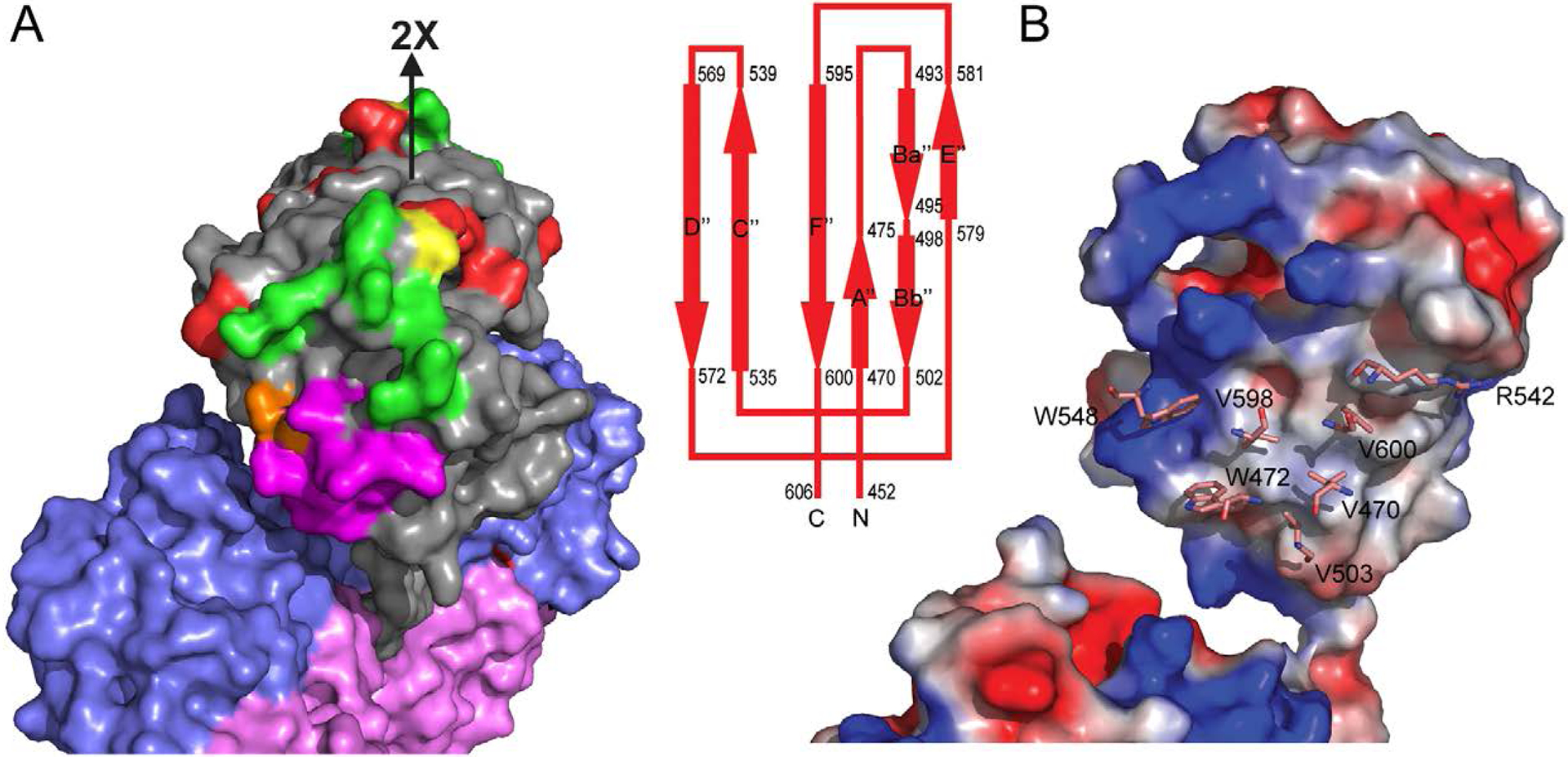
Antigenic structure and dimeric interface of the P-domain. (A) Surface presentation of a HEV dimer, in which the P-, M-, and S-domain are colored in grey, slate blue, and light pink, respectively. The antibody-binding amino acids identified by mutagenesis are colored in red. They overlap with HEP224 binding-site (green) at residue S487 (yellow) and with 8C11 binding-site (magenta) at residue D496 (colored in brown). (B) Surface potential representation of PORF2 mononer to show the dimeric contacting interface at the P-domain. The blue region is positively charged while red region is negatively charge and white region is non-polar. The amino acids of ball-and-stick mode label the residues critical to dimer formation.
