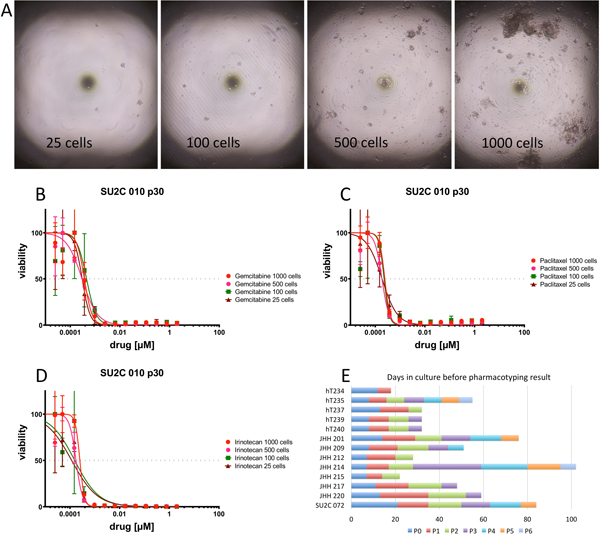
Figure 5.
Minimizing biomass needed for pharmacotyping may enable rapid drug testing in a clinical setting. (A) Photomicrograph of high-throughput culture plates containing 25, 100, 500 and 1000 cells per well. Cell survival upon exposure to (B) gemcitabine, (C) paclitaxel and (D) irinotecan with 25, 100, 500 and 1000 cells per well. (E) The interval between clinical specimen acquisition and pharmacotyping can be minimized by leveraging high-throughput technology and minimizing the biomass required for pharmacotyping. Time to pharmacotyping measured in days along x-axis for each PDO. Passage duration noted along bar graph by colors.