Abstract
PURPOSE:
Doxorubicin (DOX) is a highly effective antitumor agent widely used in cancer treatment. However, it is well established that DOX induces muscular atrophy and impairs force production. While no therapeutic interventions exist to combat DOX-induced muscle weakness, endurance exercise training has been shown to reduce skeletal muscle damage caused by DOX administration. Numerous studies have attempted to identify molecular mechanisms responsible for exercise-induced protection against DOX myotoxicity. Nevertheless, the mechanisms by which endurance exercise protects against DOX-induced muscle weakness remain elusive. In this regard, impairments to the neuromuscular junction (NMJ) are associated with muscle wasting, and studies indicate that physical exercise can rescue NMJ fragmentation. Therefore, we tested the hypothesis that exercise protects against DOX-induced myopathy by preventing detrimental changes to key proteins responsible for maintenance of the NMJ.
METHODS:
Female Sprague-Dawley rats were assigned to sedentary or exercise trained groups. Exercise training consisted of a 5-day treadmill habituation period followed by 10 days of running (60min/day, 30m/min, 0% grade). After the last training bout exercise and sedentary animals were paired with either placebo (saline) or DOX (20 mg/kg i.p.) treatment. Two days after drug treatment the soleus muscle was excised for subsequent analyses.
RESULTS:
Our results indicate that endurance exercise training prevents soleus muscle atrophy and contractile dysfunction in DOX treated animals. These adaptations were associated with increased expression of the neurotrophic factors BDNF, GNDF, NGF and NT-3. In addition, exercise enhanced the expression of Rapsyn and the acetylcholine receptor (AChR) subunits AChRβ, AChRδ and AChRγ in DOX treated animals.
CONCLUSION:
Therefore, upregulating neurotrophic factor and NMJ protein expression may be an effective strategy to prevent DOX-induced skeletal muscle dysfunction.
Keywords: chemotherapy, cardiotoxicity, anthracycline, soleus
INTRODUCTION
Doxorubicin (DOX) is a highly efficacious anthracycline antibiotic used in the treatment of a broad spectrum of human cancers including acute leukemia, lymphomas, stomach, breast and ovarian cancers (1). Unfortunately, the clinical use of DOX results in off-target effects to skeletal muscle, which adversely affect quality of life (2). Specifically, patient reported outcomes following DOX chemotherapy detail severe muscle weakness, resulting in vastly reduced work capacity and activity level (2). Studies assessing muscle weakness following DOX exposure demonstrate reduced ability to perform a 12-minute walk test (3), deficits in muscle grip strength (4) and loss of muscle mass (5). Other symptoms of skeletal muscle weakness in patients include the manifestation of dyspnea, reduced VO2max and exercise intolerance (6).
Skeletal muscle weakness occurs as a result of alterations at or distal to the neuromuscular junction (NMJ) that often persist following cessation of DOX treatment and can occur both acutely or within the years following DOX exposure (7). DOX-induced impairments to muscle force generation occurs via disruptions in redox signaling primarily as a result of its interaction with the mitochondrial respiratory chain and through a non-enzymatic reaction with ferric iron (8). Importantly, increased reactive oxygen species (ROS) in skeletal muscle results in oxidative modification to cytosolic and myofibrillar proteins and DOX-induced oxidative damage to cellular proteins triggers protein degradation via the activation of key proteolytic pathways including calpain, caspase-3, the ubiquitin proteasome system and the autophagy-lysosomal system. (9–11).
In addition to the effects of oxidative stress on cytosolic and contractile proteins, recent evidence suggests that increased skeletal muscle ROS production is sufficient to disrupt NMJ morphology (12). Specifically, Ahn et al. demonstrated that elevated mitochondrial ROS production stimulated NMJ fragmentation, degradation of the acetylcholine receptor (AChR) α subunit and reduced stability of the AChR complex, leading to reduced contractile function (12). While the direct contribution of DOX-induced mitochondrial ROS production to proteolytic signaling and skeletal muscle weakness has been described (13), the consequences to components of the NMJ have not been elucidated.
In this regard, we examined the effects of DOX on markers of NMJ function in the soleus muscle and whether exercise preconditioning promotes beneficial adaptations to NMJ proteins. Specifically, both clinical and pre-clinical investigations report that performance of regular physical activity is sufficient to reduce skeletal muscle weakness during DOX chemotherapy (14, 15). Exercise-induced adaptations to skeletal muscle function may occur due to several factors, including reductions in oxidative stress and activation of proteolytic systems (9, 10). Alternatively, exercise training has been shown to enhance the expression of several neurotrophic factors in skeletal muscle (i.e. brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), nerve growth factor (NGF) and neurotrophin-3 (NT-3)) and prevent NMJ fragmentation. Indeed, expression of these neurotrophic factors is closely tied to skeletal muscle activity and associated with maintenance of transmission between the nerve terminals and skeletal muscle fibers (16). In this regard, our results demonstrate that endurance exercise preconditioning is sufficient to reduced skeletal muscle weakness acutely following DOX administration and that the protective effects may occur as a result of the exercise-induced upregulation of neurotrophic factors, AChR subunits and proteins required for NMJ function.
METHODS
Experimental groups.
All animal experiments were approved by the University of Florida Institutional Animal Care and Use Committee. Experiments were carried out in accordance with guidelines defined by the Animal welfare act. Adult six-month old female Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were used in these experiments. Animals were maintained on a 12:12 hour reverse light-dark cycle and provided rat chow ad libitum throughout the experimental period. Rats were randomly assigned to one of four treatment groups: 1) Sedentary animals; treated with saline (SED-SALINE) (n=8/group); 2) Sedentary animals; treated with DOX (SED-DOX) (n=8/group); 3) Exercise trained animals; treated with saline (EX-SALINE) (n=7/group); and 4) Exercise trained animals; treated with DOX (EX-DOX) (n=8/group).
Exercise training protocol.
Animals assigned to the exercise trained groups performed progressive habituation training on a treadmill for 5 consecutive days (10, 20, 30, 40 and 50 min/day on days 1–5, respectively). Following two days of rest, animals then performed 10 days of treadmill running (5 days/week, 60min/day at 30m/min, 0% grade). All exercise trained animals ran for equal durations of time and covered identical distances. This exercise training protocol was implemented based on prior experiments demonstrating its ability to elicit myoprotection (9, 17). Due to an inability to complete the exercise training protocol, one animal was removed from the study. All other animals completed the exercise protocol without incident, with no noticeable difference in exercise performance, and without apparent complications. Exercise preconditioned animals were randomly assigned to either the SALINE or DOX treatment group, which was administered 24 hours after the final bout of exercise.
Doxorubicin administration.
Animals assigned to the SED-DOX and EX-DOX groups received an intraperitoneal (IP) injection of doxorubicin hydrochloride (20 mg/kg body weight) 48 hours prior to sacrifice. This dose of DOX has been scaled for use in rats and is a regularly used animal dose that is within the range used for humans (18). It is well-established that this dose is sufficient to induce skeletal muscle dysfunction in animals (19). Saline was used as the placebo treatment, and animals in the SED-SALINE and EX-SALINE groups received an IP injection of an equal volume of saline 48 hours prior to sacrifice.
Tissue harvesting.
At the conclusion of the experimental period, animals in each group were acutely anesthetized with sodium pentobarbital (60 mg/kg bodyweight IP). After reaching a surgical plane of anesthesia, each soleus muscle was carefully excised for subsequent analyses. One whole soleus muscle was used to measure in vitro contractile function. A portion from the mid-belly of the other soleus muscle was frozen in O.C.T. compound for histological analysis, and the rest of the muscle was immediately frozen in liquid nitrogen and stored at −80°C for subsequent biochemical analysis. Animals were euthanized by removal of the heart.
Histological Analysis
Myofiber cross-sectional area.
Frozen soleus sections were cut at a thickness of 10 μm with a cryostat (HM 550 Cryostat, Thermo Scientific, Waltham, MA) and stained for dystrophin (RB9027R7) (Thermo Scientific), myosin heavy chain Type I (A4.840) (Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA), and Myosin Heavy Chain Type IIa (SC-71) (DSHB) for soleus muscle fiber cross-sectional area (CSA) analysis. CSA was analyzed with Scion Image software (National Institutes of Health).
Functional analysis
Measurement of soleus muscle contractile function.
To assess soleus muscle contractile function, one whole soleus muscle was used. The muscle was suspended vertically by the tendinous attachments within a jacketed tissue bath containing 25°C Krebs-Hensleit solution equilibrated with 95% oxygen and 5% carbon dioxide gas. Muscle performance was measured as previously described (20). Specific force (N/cm2) of soleus muscles were multiplied to the ratio of fiber length to muscle weight.
Biochemical Analyses
Western blot analyses.
Soleus muscle was homogenized 1:10 (wt/vol) in 5 mM Tris (pH 7.5) and 5 mM EDTA (pH 8.0) with a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and centrifuged at 1,500 g for 10 min at 4°C. The resulting supernatant (cytosolic fraction) was collected. Soleus protein content was assessed by the method of Bradford (Sigma-Aldrich) and proteins (40 μg) were then separated by SDS-PAGE via 4–20% gradient polyacrylamide gels containing 0.1% SDS. After electrophoresis, the proteins were transferred to nitrocellulose membranes. Nonspecific sites were blocked for 2 hrs at room temperature in PBS solution containing 0.05% Tween and 5% non-fat dry milk. Membranes were incubated overnight at 4°C with primary antibodies directed against muscle-specific kinase (MuSK) (ab5619), Agrin (ab12362), low-density lipoprotein receptor-related protein 4 (LRP4) (ab174637), Dok-7 (ab75049), receptor associated protein of the synapse (Rapsyn) (ab156002), tropomyosin receptor kinase A (TrkA) (ab76291), tropomyosin receptor kinase B (TrkB) (ab18987), and nerve growth factor receptor (NGFr) (ab52987) (Abcam, Cambridge, MA). Membranes were then washed with PBS-Tween and incubated with IRDye secondary antibodies (LI-COR Biosciences, Lincoln, NE). Membranes were imaged (LI-COR Odyssey CLx) and analyzed using Image Studio Lite Ver 5.0. Membranes were stripped and re-probed for α-tubulin (DSHB), which was used to normalize for equal protein loading and transfer.
RNA isolation and cDNA synthesis.
Total RNA was isolated from muscle tissue with TRIzol Reagent (Thermo Scientific) following the manufacturer’s instructions. Total RNA and RNA content (μg/mg muscle) were evaluated by spectrophotometry. Subsequently, total RNA (5μg) was reverse transcribed with the Superscript III First-Strand Synthesis System for RT-PCR (Thermo Scientific), using oligo(dT)20 primers and the protocol outlined by the manufacturer.
Real-time polymerase chain reaction.
One microliter of cDNA was added to a 24-μl PCR reaction for real-time PCR using Taqman chemistry and the ABI Prism 7000 Sequence Detection system (ABI, Foster City, CA). Relative quantification of gene expression was performed using the comparative computed tomography method (ABI, User Bulletin no.2) as described previously (10). β-Glucuronidase, a lysosomal glycoside hydrolase, was chosen as the reference gene for soleus muscle samples based on pervious work showing unchanged expression with our experimental manipulations (21). MuSK, Agrin, LRP4, Dok-7, Rapsyn, AChRα, AChRβ, AChRδ, AChRε, AChRγ, brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF), nerve growth factor (NGF), and neurotrophin-3 (NT-3) mRNA transcripts were assayed using predesigned rat primer and probe sequences commercially available from Applied Biosystems (Assays-on-Demand).
Statistical Analysis
Between group comparisons for all dependent measures were made by two-way analysis of variance (ANOVA). When appropriate a Tukey HSD (honestly significantly different) test was performed post-hoc. Significance was established at p < 0.05 and all values are reported as mean ± standard error.
RESULTS
Exercise preconditioning attenuates DOX-induced soleus muscle weakness
No significant difference existed between experimental groups at any point during the experimental protocol (Table 1). Assessment of absolute and relative soleus muscle weight revealed an exercise effect. Specifically, soleus weight of the exercise trained animals treated with saline was significantly elevated compared to sedentary animals.
Table 1.
Differences in body weight and soleus weight between experimental groups.
| SED-SALINE | EX-SALINE | SED-DOX | EX-DOX | |
|---|---|---|---|---|
| Injection Mass (g) | 291.0 ± 6.1 | 296.7 ± 5.9 | 301.0 ± 4.9 | 287.3 ± 4.5 |
| Sacrifice Mass (g) | 285.4 ± 5.6 | 295.7 ± 4.9 | 284.6 ± 4.9 | 276.6 ± 5.9 |
| Soleus Mass (mg) | 118.5 ± 5.7 | 144.0 ± 4.2# | 117.6 ± 4.2 | 124.6 ± 4.3 |
| Soleus: Body Mass (mg/g) | 0.42 ± 0.02 | 0.49 ± 0.02# | 0.41 ± 0.02 | 0.45 ± 0.01 |
Values are presented as means ± SEM. SED = sedentary animals; EX = exercise trained animals; SALINE = saline placebo; DOX = doxorubicin treatment.
significantly different versus SED-SALINE and SED-DOX (p<0.05).
Soleus muscle fiber CSA was assessed in all experimental groups to determine the effects of exercise preconditioning and DOX administration on muscle fiber type distribution and fiber size. Analysis of fiber type distribution showed no shift in fiber type between any of the experimental groups (data not shown). Our results demonstrate that two weeks of exercise training does not affect soleus muscle fiber CSA (Figure 1). In contrast, acute DOX administration in sedentary animals revealed a main effect of DOX, which resulted in a significant reduction in soleus muscle CSA of both Type I and Type IIa fibers compared to both sedentary and exercise trained animals treated with saline. For Type IIa fibers, a main effect of exercise also was demonstrated as exercise training prior to DOX administration significantly attenuated atrophy of Type IIa muscle fibers in the soleus.
Figure 1.
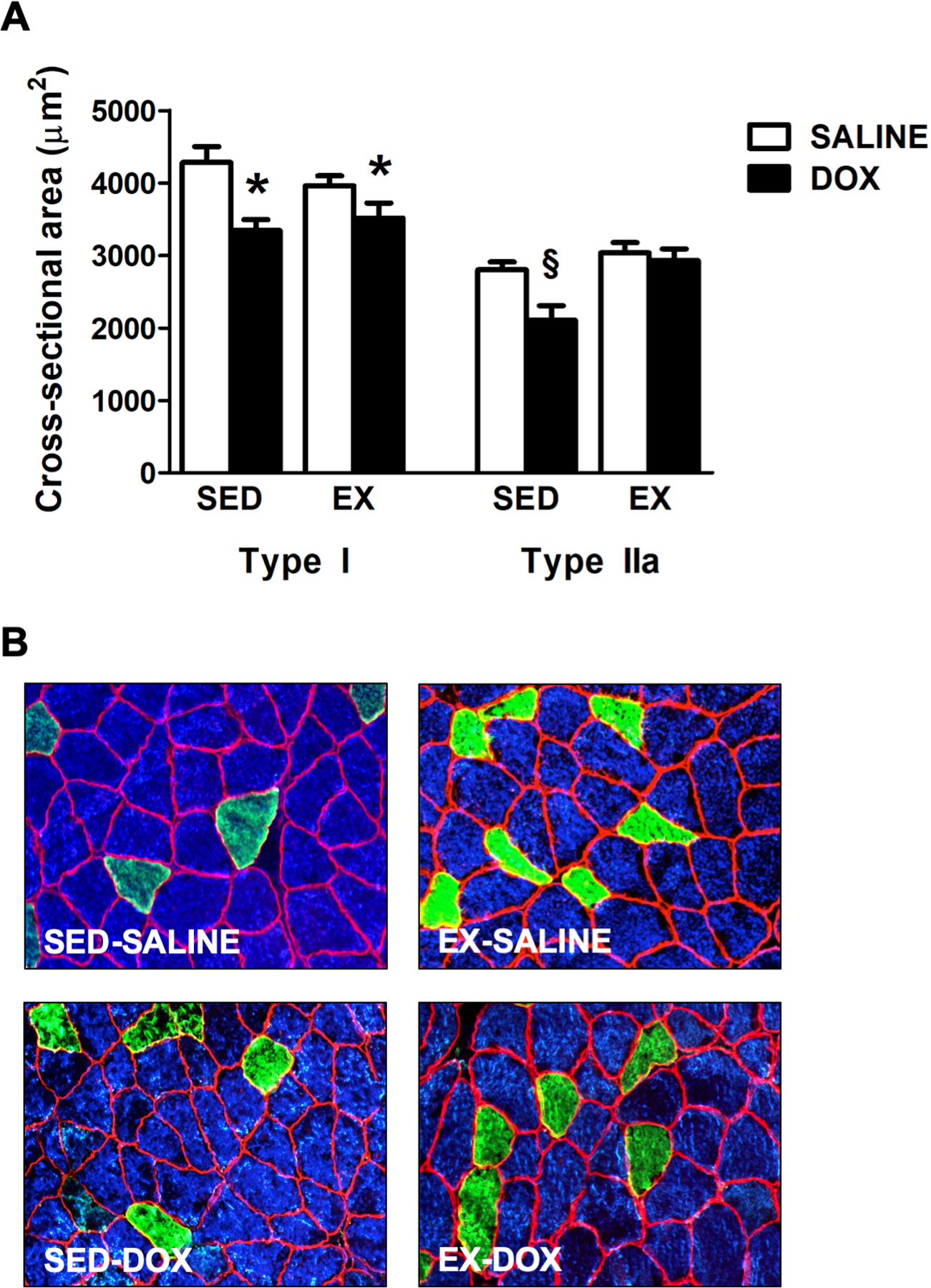
Effects of exercise preconditioning and DOX treatment on soleus muscle fiber cross-sectional area (CSA). A) Soleus CSA in sedentary (SED) and exercise trained (EX) animals treated with either SALINE or DOX. B) Representative fluorescent staining of myosin heavy chain (MHC) I (Type I) (DAPI filter / blue), MHC IIa (Type IIa) (FITC filter / green), and dystrophin (Rhodamine filter / red) proteins in soleus muscle samples. Values are mean ± SEM. * significantly different versus SED-SALINE (p<0.05). § significantly different versus all groups (p<0.05).
Previous reports indicate that exercise preconditioning is sufficient to prevent cardiorespiratory muscle dysfunction in DOX treated animals (17). Our results reveal similar findings in the soleus muscle. An exercise effect was seen at force frequencies between 30–160Hz. At 160Hz, main effects of DOX and an interaction effect were also seen. Specifically, in animals that underwent exercise preconditioning prior to DOX exposure, a contractile force production did not differ from controls at frequencies between 30–160Hz. Furthermore, soleus maximal specific force production in exercise trained animals treated with DOX was significantly preserved compared to sedentary animals treated with DOX. Conversely, sedentary animals treated with DOX demonstrate significant reductions in soleus muscle specific force production at stimulation frequencies of 30 Hz and greater (Figure 2).
Figure 2.
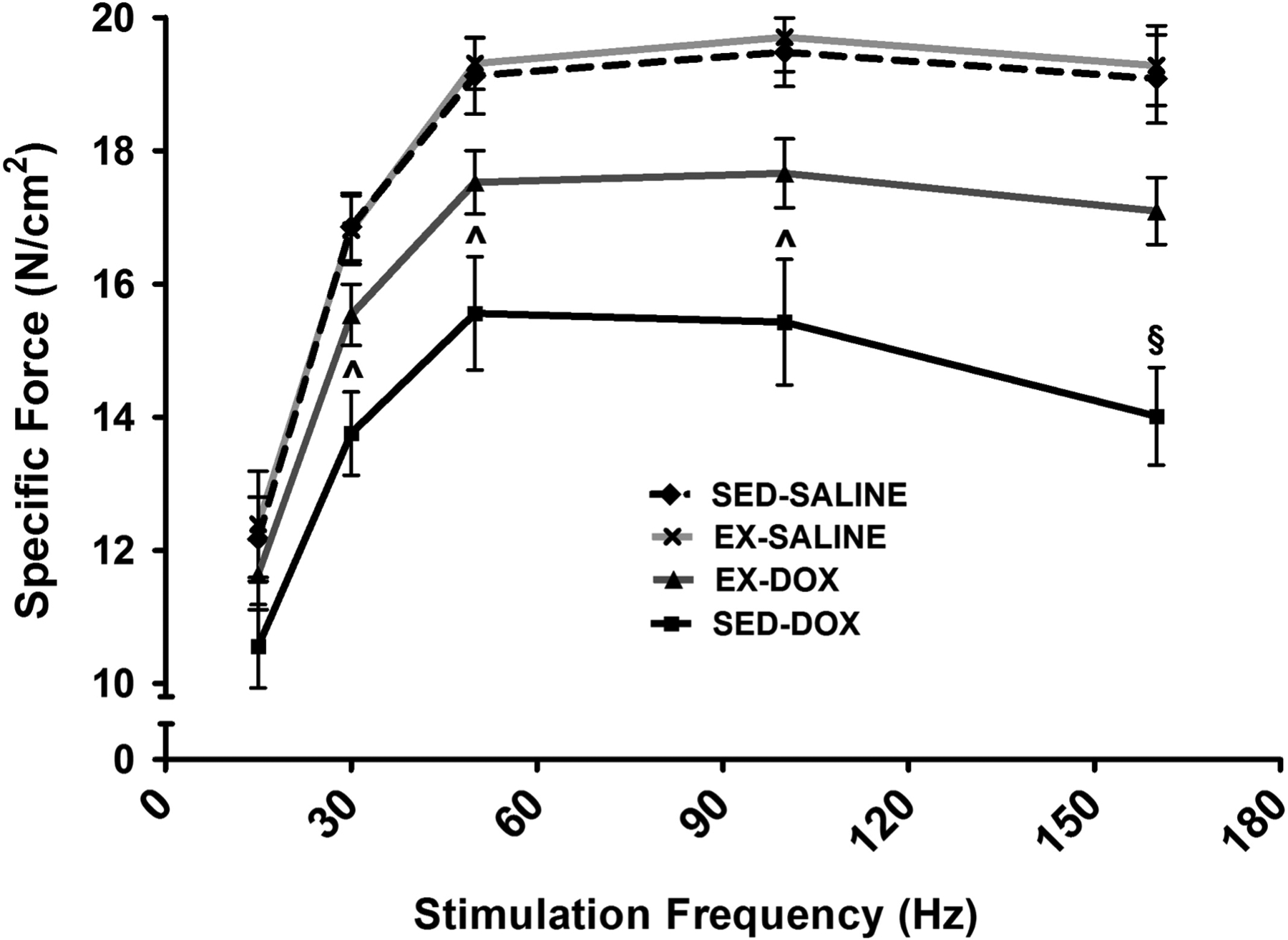
Soleus muscle force-frequency response in sedentary (SED) and exercise trained (EX) animals treated with either SALINE or DOX. Values are mean ± SEM. ^ significantly different versus SED-SALINE and EX-SALINE (p<0.05). § significantly different versus all groups (p<0.05).
DOX treatment alters neuromuscular junction protein expression
Neuromuscular synapse stability requires proper signaling through the agrin-LRP4-MuSK complex, and maintenance of the NMJ is dependent upon the agrin-mediated activation of MuSK (22). Our results show that DOX administration alters the concentration of several proteins in the soleus muscle that are involved in the agrin-LRP4-MusK signaling axis (Figure 3A–B). Measured effects for each protein include an effect of DOX for MuSK mRNA and protein expression, and an exercise effect for Agrin protein expression. Specifically, DOX treatment in sedentary animals significantly upregulated the mRNA expression of MuSK compared to sedentary animals treated with saline. MuSK protein expression was also elevated following DOX administration in sedentary animals compared to both saline treated sedentary and exercise trained animals. Agrin mRNA expression was not different between groups. However, protein expression was significantly reduced in sedentary DOX animals compared to animals that underwent exercise preconditioning prior to saline or DOX treatment. Finally, assessment of LRP4 expression demonstrated no main effects and no differences between experimental groups.
Figure 3.
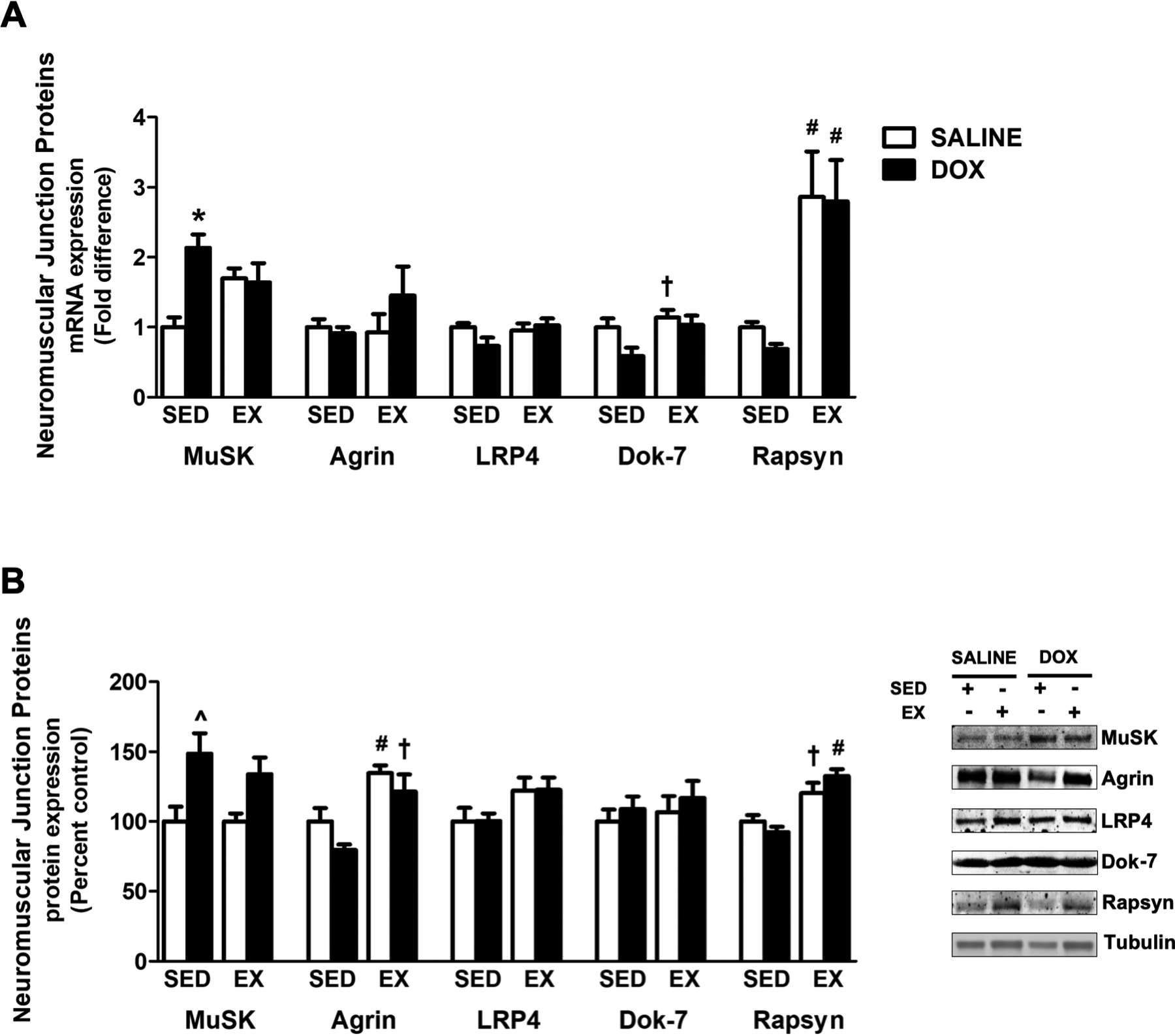
Impact of exercise preconditioning on the expression of neuromuscular junction proteins. A) mRNA expression of MuSK, Agrin, LRP4, Dok-7 and Rapsyn. B) Protein expression of MuSK, Agrin, LRP4, Dok-7 and Rapsyn. Representative western blots are pictured to the right of the graph. Tubulin was used to normalize for equal protein loading. Values are mean ± SEM. * significantly different versus SED-SALINE (p<0.05). # significantly different versus SED-SALINE and SED-DOX (p<0.05). ^ significantly different versus SED-SALINE and EX-SALINE (p<0.05). † significantly different versus SED-DOX (p<0.05).
In addition to these proteins, Dok-7 and Rapsyn expression is required at the NMJ for acetylcholine receptor (AChR) clustering (23). Assessment of Dok-7 protein expression revealed no main effects and no differences among groups. Dok-7 mRNA expression showed an exercise and DOX effect and a significant increase in exercise trained animals treated with saline compared to sedentary animals treated with DOX. Assessment of Rapsyn expression demonstrated significant effects of exercise, with mRNA expression significantly increased in response to exercise training compared to both sedentary groups. Rapsyn protein expression was increased in exercise trained animals treated with DOX compared to both sedentary groups. Additionally, exercise trained animals treated with saline also had increased Rapsyn protein expression compared to sedentary animals treated with DOX.
DOX reduces acetylcholine receptor subunit expression
AChRs are localized at the NMJ and are composed of five different subunits (α, β, δ, ε and γ) depending on AChR type (24). Previous reports demonstrate that AChR expression is reduced during conditions of skeletal muscle atrophy, and our results demonstrate that DOX-induced soleus muscle atrophy coincides with reductions in AChR subunit expression (Figure 4). Specifically, two-way analysis demonstrated a significant effect of exercise for all five subunits and a significant effect of DOX for AChRα, AChRδ and AChRγ. Post-hoc analysis indicated that mRNA expression of AChRα is significantly reduced in sedentary DOX treated animals compared to both sedentary and exercise trained animals treated with saline. Moreover, AChRδ expression is reduced in sedentary DOX treated animals compared to all other groups, AChRβ was significantly enhanced as a result of exercise with mRNA expression elevated in both exercise trained groups compared to both sedentary groups, and AChRγ was elevated in both exercise groups compared to sedentary animals treated with DOX. Finally, no significant changes were found in the measurement of mRNA expression of AChRε.
Figure 4.
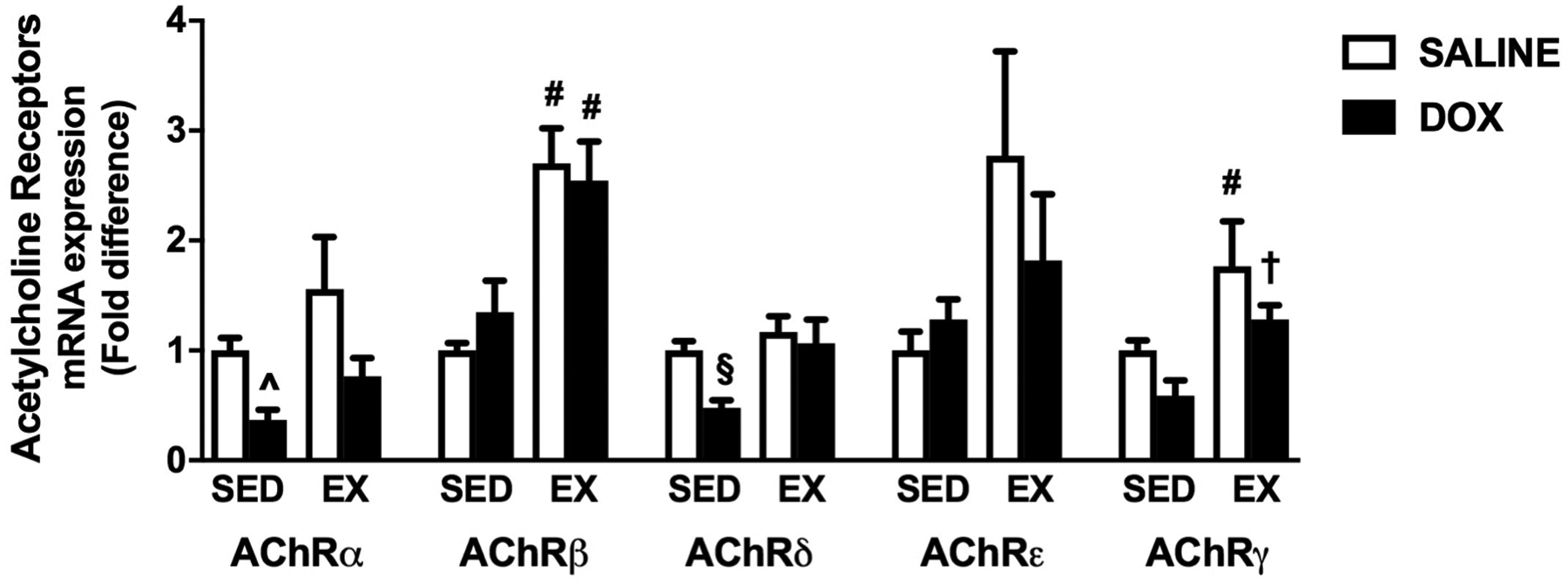
Effects of exercise preconditioning acetylcholine receptor (AChR) expression. mRNA expression of AChRα, AChRβ, AChRδ, AChRε and AChRγ. Values are mean ± SEM. # significantly different versus SED-SALINE and SED-DOX (p<0.05). ^ significantly different versus SED-SALINE and EX-SALINE (p<0.05). § significantly different versus all groups (p<0.05). † significantly different versus SED-DOX (p<0.05).
Exercise training upregulates neurotrophic factor expression in the soleus
Physical activity has been demonstrated to induce an increase in the expression of neurotrophic factors in skeletal muscle (25). Our results confirm this as main effects for exercise were shown for the neurotrophic factors BDNF, GDNF and NGF. Two-way analysis for NT-3 revealed an effect of both exercise and DOX treatment. Indeed, the mRNA expression of BDNF was significantly increased with exercise training compared to sedentary animals (Figure 5A). Additionally, upregulation of BDNF in the soleus of exercise trained animals occurred independent of DOX or saline treatment. Exercise also elicited an increase in GNDF, NGF and NT-3 mRNA expression compared to sedentary animals treated with DOX. Finally, while exercise effects were revealed for TrkB, post hoc analysis revealed no significant differences in the expression of TrkA, TrkB or NGFr protein expression between groups (Figure 5B).
Figure 5.
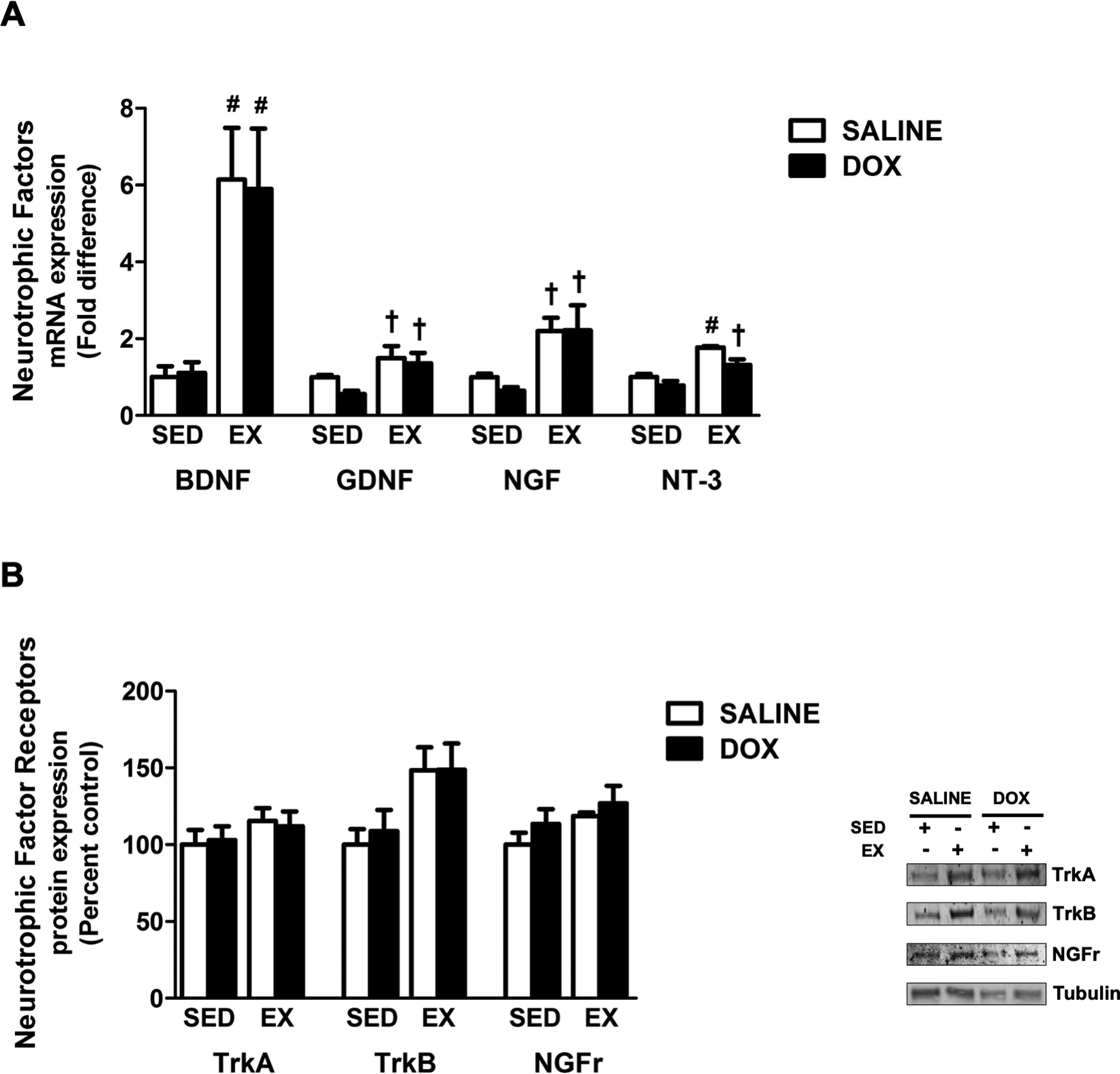
Effects of exercise and DOX on the expression of neurotrophic factors and their receptors. A) mRNA expression of BDNF, GDNF, NGF and NT-3. B) Protein expression of TrkA, TrkB and NGFr. Representative western blots are pictured to the right of the graph. Tubulin was used to normalize for equal protein loading. Values are mean ± SEM. # significantly different versus SED-SALINE and SED-DOX (p<0.05). † significantly different versus SED-DOX (p<0.05).
DISCUSSION
The adverse physiological effects of anti-cancer therapy extend to the musculoskeletal system and limit patient quality of life as a result of persistent muscle weakness (13). Specifically, DOX chemotherapy results in the onset of muscular weakness in patients, which is associated with severe reductions in muscle fiber cross-sectional area and neuromuscular toxicity following treatment (5). These findings in patients validate preclinical reports, which demonstrate reduced time to fatigue, reduced specific force production and muscle fiber atrophy following DOX treatment (14, 17, 26, 27). Indeed, Doroshow and colleagues were among the first to discover that DOX toxicity extends beyond cardiomyopathy when they revealed significant ultrastructural injury and myofibrillar disorganization of the diaphragm and gastrocnemius muscle in mice following a single intraperitoneal injection of DOX (28). Thus, it is now well established that DOX potentiates reductions in both submaximal and maximal tetanic force production in both slow and fast twitch muscles, with slow twitch muscles exhibiting greater deficits (27). Our results confirm that acute DOX administration significantly reduces soleus muscle contractile force production and also causes significant muscle fiber atrophy.
Given the toxic effects of DOX on skeletal muscle structure and function, it is necessary to develop interventions that successfully prevent muscle dysfunction while also retaining the anti-tumor efficacy of DOX. In this regard, exercise training prior to or during DOX treatment can improve muscle force production, reduce fatigability and prevent skeletal muscle atrophy (14, 17, 26). Interestingly, exercise-induced protection against skeletal muscle toxicity does not appear to be specific to the exercise prescription as high-intensity interval training (26) and 10-weeks of endurance exercise (14) both promote beneficial adaptations. Additionally, our laboratory reported improved muscle specific force production and increased muscle fiber diameter in the diaphragm of DOX treated animals following two weeks of exercise preconditioning (17), and results from the current study reveal comparable modifications to soleus muscle. Importantly, investigation into the effects of DOX on tumor growth reveals that exercise provides cytoprotection to the heart and skeletal muscle, and does not affect DOX accumulation within tumor cells or the degree of tumor shrinkage (29).
DOX alters NMJ protein expression in the soleus muscle
Physical activity is an effective intervention to maintain muscle mass and function when combined with DOX chemotherapy (14, 17, 26). However, the mechanisms for this protection are not well understood. During conditions of skeletal muscle atrophy, exercise has been shown to maintain muscle function through adaptations to the NMJ, which can induce NMJ hypertrophy via direct modification and by increasing muscle fiber size (30). Furthermore, in healthy muscle, exercise training is reported to increase the number of acetylcholine (ACh)-containing vesicles, the expression of acetylcholinesterase and the number of AChRs within the motor endplate (31). Exercise can also augment MuSK activation via increased agrin expression and binding, resulting in stabilization of the NMJ and clustering of AChRs in the motor endplate (32). Hence, physical activity is associated with increased resistance to fatigue and increased muscle strength as a result of improved maintenance and regeneration of the NMJ (33).
Beneficial modifications to the NMJ following exercise are thought to occur, in part, as a result of increased peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α) expression (34). PGC-1α is a transcriptional coactivator in skeletal muscle, and evidence shows that PGC-1α can regulate NMJ formation and gene expression (34). Specifically, work in transgenic animals determined that muscle-specific knock out of PGC-1α induces a reduction in AChR subunit expression, whereas overexpression of PCG-1α stimulates a significant increase in the gene expression of the AChR subunits and Rapsyn (34). DOX and exercise-induced changes in NMJ gene expression may be linked to these findings as DOX treatment has been demonstrated to reduce PGC-1α expression (35). In addition, our group has demonstrated that short-term endurance exercise training prior to DOX administration is sufficient to upregulate PGC-1α expression in the soleus muscle (36). Our results correlate with these findings as DOX treatment elicited a reduction in AChRα and AChRδ expression. Further, endurance exercise preconditioning elicited increased gene expression of AChRα, AChRβ, AChRδ, AChRγ and Rapsyn compared to sedentary animals treated with DOX.
Exercise training enhances soleus neurotrophic factor expression
Neurotrophic factor expression is associated with beneficial adaptations to neuronal tissue that enhance axonal branching, transmission of electrical signals and cell survival during pathological conditions (37). In addition, recent evidence suggests that neurotrophic factor expression not only positively regulates motoneuron function, but it can also enhance muscular function and protein synthesis (38). Significantly, exercise training has been shown to modulate the structure and function of the NMJ by increasing the size and amount of branching within the motor nerve terminal and increasing ACh release (31). Increased muscular activity can also elicit increased expression of several neurotrophic factors in skeletal muscle (e.g BDNF, GDNF, NGF and NT-3) (25). These changes in neurotrophic factor expression are associated with the observed beneficial modifications to the NMJ, and are also related to anabolic signaling within the skeletal muscle (37).
While the potential for each neurotrophic factor to elicit protection against DOX skeletal muscle toxicity has not been determined, treatment with exogenous BDNF in cardiac muscle is sufficient to ameliorate DOX-induced reductions in cardiac ejection fraction and fractional shortening percentage (39). Further, in the skeletal muscle of heart failure animals, exercise-induced increases in BDNF expression elicit anti-atrophic effects (38). During these conditions, the beneficial effects of BDNF were associated with increased Akt signaling, resulting in reduced apoptosis and improved antioxidant capacity (38, 39). Additionally, contraction-induced BDNF expression has been shown to evoke increased ACh release from the NMJ, which can stimulate enhancement of muscle contractile force (40). Therefore, the finding that two-weeks of exercise training induced a significant increase in soleus muscle BDNF expression could indicate its importance in facilitating a protective phenotype in exercise trained animals treated with DOX.
Limitations to the study
The experimental design used in this study provides a controlled pre-clinical model to assess the molecular pathways altered by both DOX and exercise training. While the primary goal was to determine the mechanism by which exercise can mitigate DOX-induced skeletal muscle dysfunction, several points should be considered. First, although the bolus dose of DOX has been a commonly utilized treatment protocol to assess the signaling pathways that promote skeletal muscle weakness, this does not replicate the clinical setting in which DOX is typically administered over several cycles (18). Second, while the majority of studies investigating DOX muscle toxicity use male animals, these experiments utilized female animals. Previous reports from our group have demonstrated similar changes in skeletal muscle signaling between sexes. Further studies will be needed to determine if similar findings are revealed between male and female rats. Additionally, the authors chose to only evaluate the soleus muscle based on studies demonstrating a more profound effect of DOX on slow-twitch muscles. However, the inability to compare changes to different muscle types is a limitation to the study. Finally, while our data support the hypothesis that exercise preconditioning elicits beneficial adaptations to the skeletal muscle as a result of enhanced neurotrophic factor expression and through enhancing NMJ and AChR protein expression future work needs to be done to assess NMJ integrity/structure and function.
CONCLUSION
Endurance exercise training is a beneficial therapeutic intervention that can be used to combat DOX-induced skeletal muscle dysfunction and weakness. Clinical evidence continues to demonstrate that participation in regular physical activity during DOX chemotherapy is sufficient to reduce muscle weakness (15). While these are meaningful findings, prescribing exercise to patients is limited in the setting of restrictive or advanced disease state. Therefore, it is necessary to use exercise as a tool to determine cytoprotective proteins that can be manipulated within the skeletal muscle to reduce weakness while maintaining the anti-neoplastic properties of DOX. In this regard, the current study demonstrates that short-term endurance exercise training is sufficient to prevent DOX-induced skeletal muscle function and that these effects may be the result of beneficial adaptations to NMJ structure and function. In particular, the primary effects of exercise appear to be related to increased neurotrophic factor expression which may protect against skeletal muscle dysfunction by enhancing NMJ function. Alternatively, these effects may be mediated by positive regulation of Akt signaling to increase protein synthesis and prevent proteolytic breakdown of the muscle. Future experiments are needed to determine the precise role of exercise-induced expression of BDNF, GDNF, NGF and NT-3 in the soleus muscle following DOX administration.
ACKNOWLEDGEMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. ABM was supported by NIH T32 HD043730.
Footnotes
CONFLICT OF INTEREST
No conflicts of interest, financial or otherwise, are declared by the authors. The results of the present study do not constitute endorsement by ACSM, and are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
REFERENCES
- 1.Carter SK. Adriamycin-a review. J Natl Cancer Inst. 1975;55(6):1265–74. [DOI] [PubMed] [Google Scholar]
- 2.Elbl L, Vasova I, Tomaskova I et al. Cardiopulmonary exercise testing in the evaluation of functional capacity after treatment of lymphomas in adults. Leuk Lymphoma. 2006;47(5):843–51. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz AL, Winters-Stone K, Gallucci B. Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncol Nurs Forum. 2007;34(3):627–33. [DOI] [PubMed] [Google Scholar]
- 4.Wright MJ, Halton JM, Martin RF, Barr RD. Long-term gross motor performance following treatment for acute lymphoblastic leukemia. Med Pediatr Oncol. 1998;31(2):86–90. [DOI] [PubMed] [Google Scholar]
- 5.Bonifati DM, Ori C, Rossi CR, Caira S, Fanin M, Angelini C. Neuromuscular damage after hyperthermic isolated limb perfusion in patients with melanoma or sarcoma treated with chemotherapeutic agents. Cancer Chemother Pharmacol. 2000;46(6):517–22. [DOI] [PubMed] [Google Scholar]
- 6.Peel AB, Thomas SM, Dittus K, Jones LW, Lakoski SG. Cardiorespiratory fitness in breast cancer patients: a call for normative values. J Am Heart Assoc. 2014;3(1):e000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meeske K, Smith AW, Alfano CM et al. Fatigue in breast cancer survivors two to five years post diagnosis: a HEAL Study report. Qual Life Res. 2007;16(6):947–60. [DOI] [PubMed] [Google Scholar]
- 8.Horenstein MS, Vander Heide RS, L’Ecuyer TJ. Molecular basis of anthracycline-induced cardiotoxicity and its prevention. Mol Genet Metab. 2000;71(1–2):436–44. [DOI] [PubMed] [Google Scholar]
- 9.Smuder AJ, Kavazis AN, Min K, Powers SK. Exercise protects against doxorubicin-induced oxidative stress and proteolysis in skeletal muscle. J Appl Physiol (1985). 2011;110(4):935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smuder AJ, Kavazis AN, Min K, Powers SK. Exercise protects against doxorubicin-induced markers of autophagy signaling in skeletal muscle. J Appl Physiol (1985). 2011;111(4):1190–8. [DOI] [PubMed] [Google Scholar]
- 11.Smuder AJ, Kavazis AN, Hudson MB, Nelson WB, Powers SK. Oxidation enhances myofibrillar protein degradation via calpain and caspase-3. Free Radic Biol Med. 2010;49(7):1152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahn B, Ranjit R, Premkumar P et al. Mitochondrial oxidative stress impairs contractile function but paradoxically increases muscle mass via fibre branching. J Cachexia Sarcopenia Muscle. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilliam LA, St Clair DK. Chemotherapy-induced weakness and fatigue in skeletal muscle: the role of oxidative stress. Antioxid Redox Signal. 2011;15(9):2543–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bredahl EC, Pfannenstiel KB, Quinn CJ, Hayward R, Hydock DS. Effects of Exercise on Doxorubicin-Induced Skeletal Muscle Dysfunction. Med Sci Sports Exerc. 2016;48(8):1468–73. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz AL, Mori M, Gao R, Nail LM, King ME. Exercise reduces daily fatigue in women with breast cancer receiving chemotherapy. Med Sci Sports Exerc. 2001;33(5):718–23. [DOI] [PubMed] [Google Scholar]
- 16.Krause Neto W, Ciena AP, Anaruma CA, de Souza RR, Gama EF. Effects of exercise on neuromuscular junction components across age: systematic review of animal experimental studies. BMC Res Notes. 2015;8:713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morton AB, Mor Huertas A, Hinkley JM, Ichinoseki-Sekine N, Christou DD, Smuder AJ. Mitochondrial accumulation of doxorubicin in cardiac and diaphragm muscle following exercise preconditioning. Mitochondrion. 2019. 45:52–62;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiensch AE, Bolam KA, Mijwel S et al. Doxorubicin-induced skeletal muscle atrophy: Elucidating the underlying molecular pathways. Acta Physiol (Oxf). 2019:e13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilliam LA, Moylan JS, Callahan LA, Sumandea MP, Reid MB. Doxorubicin causes diaphragm weakness in murine models of cancer chemotherapy. Muscle Nerve. 2011;43(1):94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts BM, Frye GS, Ahn B, Ferreira LF, Judge AR. Cancer cachexia decreases specific force and accelerates fatigue in limb muscle. Biochem Biophys Res Commun. 2013;435(3):488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deruisseau KC, Kavazis AN, Powers SK. Selective downregulation of ubiquitin conjugation cascade mRNA occurs in the senescent rat soleus muscle. Exp Gerontol. 2005;40(6):526–31. [DOI] [PubMed] [Google Scholar]
- 22.Zong Y, Jin R. Structural mechanisms of the agrin-LRP4-MuSK signaling pathway in neuromuscular junction differentiation. Cell Mol Life Sci. 2013;70(17):3077–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beeson D, Higuchi O, Palace J et al. Dok-7 mutations underlie a neuromuscular junction synaptopathy. Science. 2006;313(5795):1975–8. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Sugiura Y, Padgett D, Lin W. Postsynaptic development of the neuromuscular junction in mice lacking the gamma-subunit of muscle nicotinic acetylcholine receptor. J Mol Neurosci. 2010;40(1–2):21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dupont-Versteegden EE, Houle JD, Dennis RA et al. Exercise-induced gene expression in soleus muscle is dependent on time after spinal cord injury in rats. Muscle Nerve. 2004;29(1):73–81. [DOI] [PubMed] [Google Scholar]
- 26.Dickinson JM, D’Lugos AC, Mahmood TN et al. Exercise Protects Skeletal Muscle during Chronic Doxorubicin Administration. Med Sci Sports Exerc. 2017;49(12):2394–403. [DOI] [PubMed] [Google Scholar]
- 27.Ertunc M, Sara Y, Korkusuz P, Onur R. Differential contractile impairment of fast- and slow-twitch skeletal muscles in a rat model of doxorubicin-induced congestive heart failure. Pharmacology. 2009;84(4):240–8. [DOI] [PubMed] [Google Scholar]
- 28.Doroshow JH, Tallent C, Schechter JE. Ultrastructural features of Adriamycin-induced skeletal and cardiac muscle toxicity. Am J Pathol. 1985;118(2):288–97. [PMC free article] [PubMed] [Google Scholar]
- 29.Parry TL, Hayward R. Exercise training does not affect anthracycline antitumor efficacy while attenuating cardiac dysfunction. Am J Physiol Regul Integr Comp Physiol. 2015;309(6):R675–83. [DOI] [PubMed] [Google Scholar]
- 30.Nishimune H, Stanford JA, Mori Y. Role of exercise in maintaining the integrity of the neuromuscular junction. Muscle Nerve. 2014;49(3):315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deschenes MR. Adaptations of the neuromuscular junction to exercise training. Current Opinion in Physiology. 2019;10:10–6. [Google Scholar]
- 32.Bahreinipour MA, Joukar S, Hovanloo F et al. Mild aerobic training with blood flow restriction increases the hypertrophy index and MuSK in both slow and fast muscles of old rats: Role of PGC-1alpha. Life Sci. 2018;202:103–9. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Freire M, de Cabo R, Studenski SA, Ferrucci L. The Neuromuscular Junction: Aging at the Crossroad between Nerves and Muscle. Front Aging Neurosci. 2014;6:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Handschin C, Kobayashi YM, Chin S, Seale P, Campbell KP, Spiegelman BM. PGC-1alpha regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes Dev. 2007;21(7):770–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo J, Guo Q, Fang H et al. Cardioprotection against doxorubicin by metallothionein Is associated with preservation of mitochondrial biogenesis involving PGC-1alpha pathway. Eur J Pharmacol. 2014;737:117–24. [DOI] [PubMed] [Google Scholar]
- 36.Kavazis AN, Smuder AJ, Powers SK. Effects of short-term endurance exercise training on acute doxorubicin-induced FoxO transcription in cardiac and skeletal muscle. J Appl Physiol (1985). 2014;117(3):223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakuma K, Yamaguchi A. The recent understanding of the neurotrophin’s role in skeletal muscle adaptation. J Biomed Biotechnol. 2011;2011:201696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, Wang B, Fei A. BDNF contributes to the skeletal muscle anti-atrophic effect of exercise training through AMPK-PGC1alpha signaling in heart failure mice. Arch Med Sci. 2019;15(1):214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hang P, Zhao J, Sun L et al. Brain-derived neurotrophic factor attenuates doxorubicin-induced cardiac dysfunction through activating Akt signalling in rats. J Cell Mol Med. 2017;21(4):685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santafe MM, Garcia N, Tomas M et al. The interaction between tropomyosin-related kinase B receptors and serine kinases modulates acetylcholine release in adult neuromuscular junctions. Neurosci Lett. 2014;561:171–5. [DOI] [PubMed] [Google Scholar]


