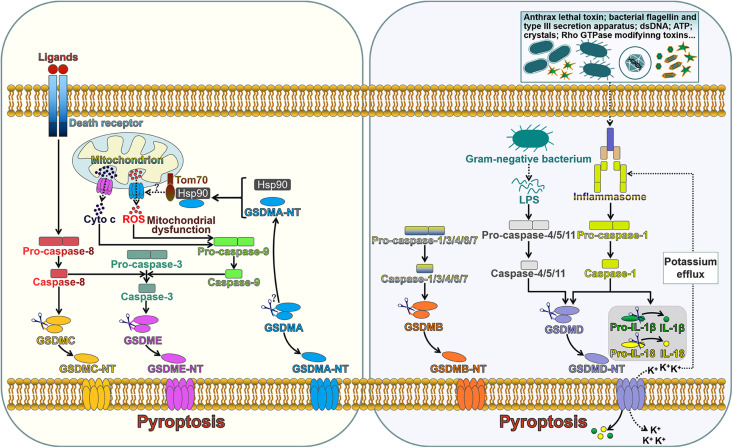
FIGURE 1.
The role of gasdermins in pore formation and pyroptotic cell death. The extrinsic pathway of apoptosis is triggered by interaction between death ligands and death receptors. The death receptor-mediated process involves the assembly of the death-inducing signaling complex (DISC) that induces the activation of caspase-8. GSDMC is cleaved into two fragments by caspase-8, generating an N-terminal functional domain and a C-terminal repressor domain. The released GSDMC-NT exerts its pore-forming effect. Caspase-8 can cleave caspase-3. Active caspase-3 proteolytically processes GSDME, producing GSDME-NT to form pores on the cell membrane and induce pyroptosis. GSDME-NT is able to permeabilize the mitochondrial membrane that leads to the leakage of cyto c. The translocation of cyto c to the cytosol can induce the activation of caspase-9 that processes pro-caspase-3 into active caspase-3. These events ultimately augment the apoptotic signaling cascade. GSDMA3-NT binds membrane lipids to form pores and triggers pyroptotic cell death. GSDMA3-NT combines with the cytosolic chaperone Hsp90 for mitochondrial targeting. Hsp90 escorts GSDMA3-NT to the mitochondrial transport receptor Tom70, leading to the generation of ROS. Mitochondrial dysfunction can result in the activation of caspase-9, which then processes pro-caspase-3 into caspase-3. GSDMB can be cleaved by caspase-1, -3, -4, -6, and -7. The unmasked N-terminal domain punches massive holes in the cell membrane. The canonical inflammasome sensors recognize various DAMPs and PAMPs and activate caspase-1. Upon activation, caspase-1 cleaves GSDMD, releasing the N-terminal effector domain from the C-terminal inhibitory domain. GSDMD-NT interacts with phosphoinositides residing on the inner leaflet of the cell membrane and oligomerizes to generate membrane pores. This event leads to cell lysis and the release of intracellular contents. Caspase-1 also cleaves the inactive proforms of IL-1β and IL-18 to generate biologically active cytokines. GSDMD-NT pores serve as a conduit for the passage of mature IL-1β and IL-18. Alternatively, caspase-4, -5, and -11 are activated by sensing intracellular LPS. Active caspase-4, -5, and -11 cleave GSDMD within the linker domain. GSDMD-NT pores mediate potassium efflux, which in turn activates the canonical inflammasome signaling. Tom70, translocase of mitochondrial outer membrane 70; Hsp90, heat shock protein 90; cyto c, cytochrome c; ROS, reactive oxygen species; dsDNA, double-stranded DNA; ATP, adenosine triphosphate; LPS, lipopolysaccharide; GSDMA-E, gasdermin A-E; GSDMA-E-N T, the N-terminal domain of GSDMA-E; IL-1β, interleukin-1β; IL-18, interleukin-18.