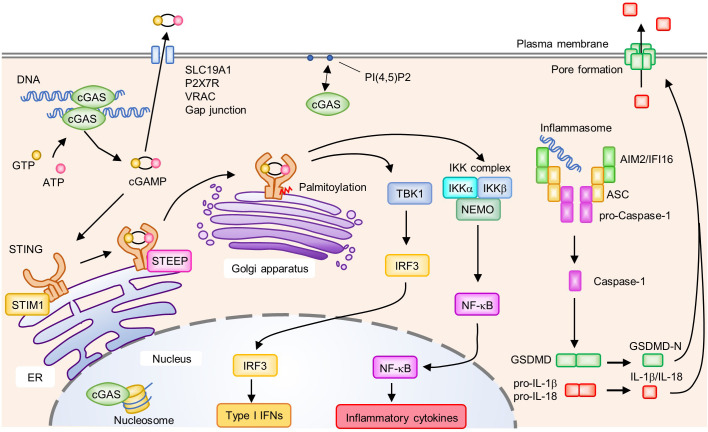
Figure 3.
Activation of the cyclic GMP-AMP (cGAMP) synthase (cGAS)-stimulator of IFN genes (STING) signaling pathway and AIM2/IFI16 inflammasome. cGAS recognizes DNA in the cytosol and subsequently synthesizes the second messenger cGAMP from GTP and ATP. In the steady state, STIM1 interacts with STING to retain it on the endoplasmic reticulum (ER) membrane. Binding of cGAMP to STING induces the translocation of STING from the ER to the Golgi apparatus. Upon activation, enhanced interactions between STEEP and STING promote the trafficking of STING from the ER to the Golgi apparatus where it undergoes post-translational modifications such as palmitoylation. Activated STING on the Golgi apparatus recruits and activates TBK1 and the IKK complex (IKKα, IKKβ, and NEMO), which induce the production of type I IFNs and proinflammatory cytokines by IRF3 and NF-κB, respectively. cGAMP activates surrounding cells by being transferred to the extracellular space via SLC19A1, P2X7R, VRAC, and gap junctions. cGAS is associated with PI(4,5)P2 on the plasma membrane and is trafficked away from the nucleus to prevent the aberrant activation of cGAS by self-derived DNA. cGAS is also localized in the nucleus where its activity is inhibited by interactions with the nucleosome. Cytosolic DNA is recognized by ALRs, leading to the formation of an inflammasome composed of AIM2 or IFI16, ASC, and pro-Caspase-1. Within the inflammasome, Caspase-1 is activated by proteolytic cleavage from pro-Caspase-1 to Caspase-1. Activated Caspase-1 cleaves GSDMD, pro-IL-1β; and pro-IL-18. The N-terminus of GSDMD (GSDMD-N) forms a pore on the plasma membrane and induces cell death accompanied by the release of biologically active IL-1β and IL-18.