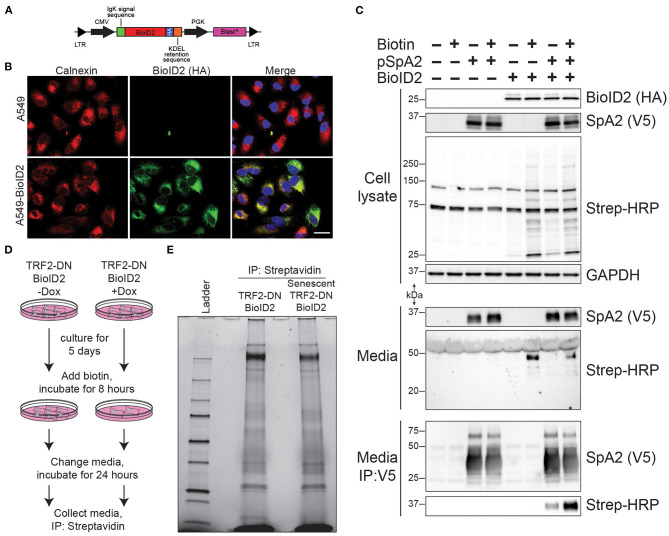
Figure 3.
ER-targeted BioID2 to label secreted proteins. (A) Schematic of lentivirus expressing ER-targeted BioID2 construct. (B) Photomicrographs of A549 and A549-BioID2 cells showing co-localization of the BioID2 (HA-tagged; green) and the ER marker calnexin (red). Nuclei are stained with DAPI (blue). Scale bar is 25 microns. (C) Proof of concept demonstrating that secreted proteins are biotinylated by ER-targeted BioID2. A549 or A549 cells stable expression ER-BioID2 were transfected with a plasmid encoding a V5-SFTPA2 construct. Eighteen hours after transfection, biotin was added to some samples and the media and cell lysates were examined the next day by western blotting for SFTPA2 (V5), BioID2 (HA), or biotinylated proteins (Strep-HRP). GAPDH was a load control for cell lysates. Biotinylated proteins were detected in the media only in cells that expressed ER-BioID2 and were cultured in excess biotin. Biotinylated SFTPA2 was detected in media only when ER-BioID2 was present. (D) Experimental procedure for unbiased proteomic analysis of secreted proteins. Cells were cultured for 5 days in the presence of Dox to induce senescence followed by incubation with excess biotin for 8 h. After biotin labeling, cells were washed and fresh media was added. Twenty-four hours later, media was collected and biotinylated proteins were isolated by incubation with streptavidin-coated beads and analyzed by stain-free SDS-page (E).