Fig. 1.
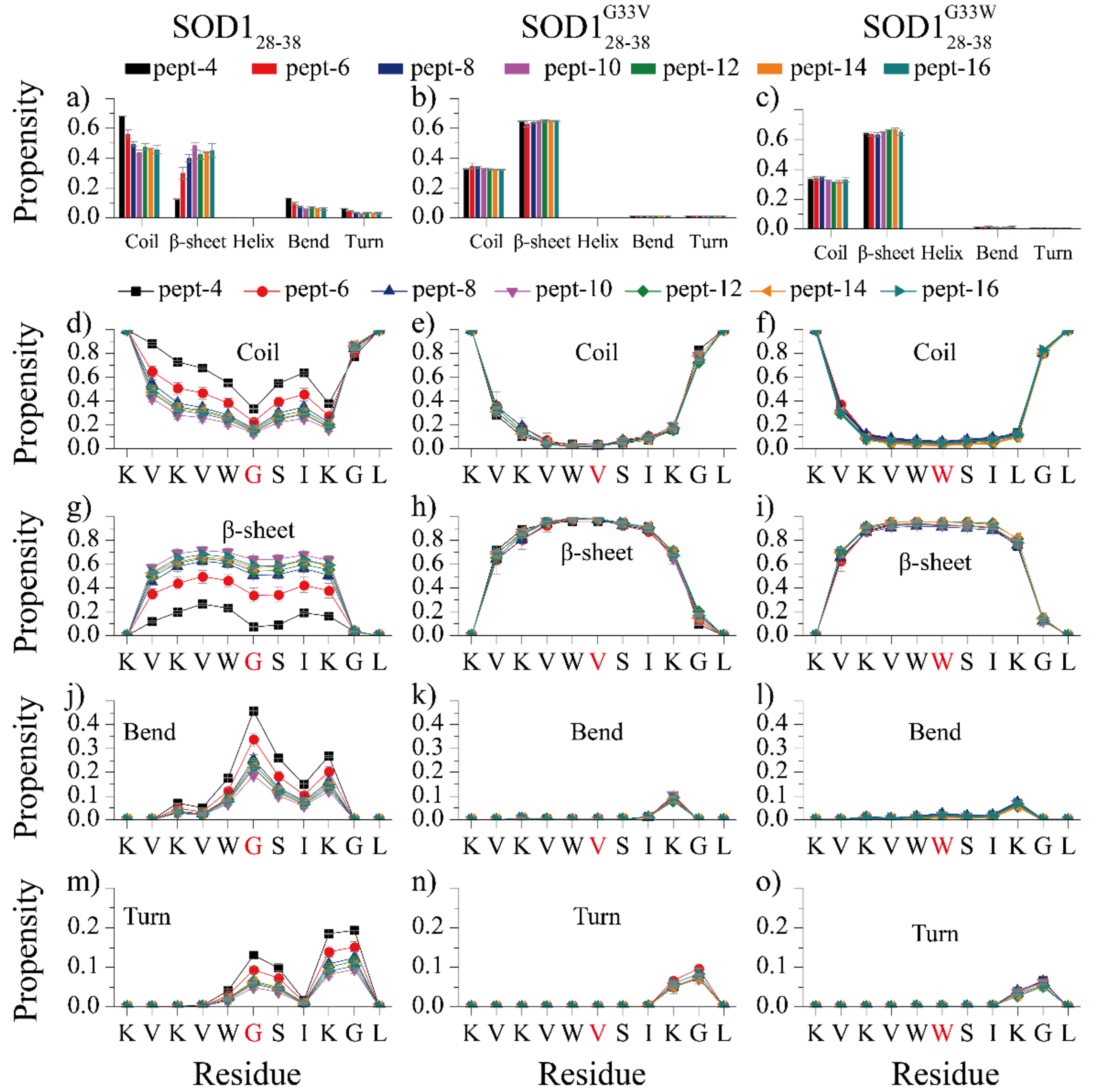
Secondary structure analyses of SOD128–38, SOD128–38G33V and SOD128–38G33W self-assemblies, (a-c) The average secondary structure contents in terms of coil, β-sheet, helix, bend and turn for SOD128–38, SOD128–38G33V and SOD128–38G33W aggregation with different number of peptides. The propensity of each residue adopted coil (d-f), β-sheet (g-i), bend (j-k) and turn (m-o) for each type of peptides with different system sizes. The error bars of secondary structure propensities correspond to the standard deviations of means from 20 independent simulations. Our results suggest that substituting flexible glycine at residue position 33 (highlighted in red in panels d-o) with hydrophobic valine and tryptophan significantly increased the β-sheet propensity in the aggregates.
