Abstract
Objective:
Hyperglycemia is a common comorbidity for ischemic stroke, and is associated with worsened neurological outcomes. Platelets are central mediators of ischemic stroke and hyperglycemia mediates platelet hyperactivity. In this study, we investigated the contribution of platelet glucose metabolism to ischemic stroke.
Methods:
Mice lacking both Glut1 and Glut3 specifically in platelets (DKO) and their littermate controls (WT) were subjected to one-hour transient middle cerebral artery occlusion under normoglycemic and streptozotocin-induced hyperglycemic conditions after which, stroke volume, platelet activation, and platelet-neutrophil aggregate (PNA) formation were examined.
Results:
Under normoglycemic conditions, DKO mice were protected from ischemic stroke with smaller brain infarct volumes and improved cerebral blood flow. In addition, DKO mice had reduced platelet activation, PNAs, and cerebral neutrophil recruitment after stroke. Hyperglycemia significantly increased infarct size and cerebral Evans blue extravasation and worsened neurological outcomes and cerebral blood flow in both WT and DKO mice, abolishing the protective effect witnessed under normoglycemic conditions. Flow cytometric analysis after stroke demonstrated increased platelet activation and neutrophil trafficking to the brain, independent of platelet glucose metabolism. Finally, platelets from healthy DKO mice were unable to become procoagulant upon dual agonist stimulation. Conversely, hyperglycemia increased platelet mitochondrial ROS production which potentiated procoagulant platelet formation in WT mice and restored procoagulant platelet formation in DKO mice.
Conclusion:
Hyperglycemia aggravates ischemic stroke outcome independent of platelet glucose uptake. Furthermore, we demonstrated that hyperglycemia primes procoagulant platelet formation. This underlines the therapeutic potential for strategies targeting procoagulant platelet formation for the treatment of acute ischemic stroke.
Keywords: Hyperglycemia, Stroke, Platelets, Thrombosis, Neutrophils
Introduction
Hyperglycemia is one of the most common comorbidities in ischemic stroke patients and it is associated with brain infarct growth, hemorrhagic transformation and worsened neurological outcomes.[1,2] Several experimental and clinical stroke studies have established thromboinflammation as a key mediator of ischemic stroke brain damage.[3] Interestingly, hyperglycemia facilitates thromboinflammation by activating the endothelium, platelets and neutrophils. In the setting of stroke, this was shown to impair post-stroke cerebral blood flow, disrupt the blood–brain barrier, and cause hemorrhagic transformation.[4,5] Yet, the exact mechanisms underlying these observations are incompletely understood.
Recently, we demonstrated a critical role for procoagulant platelets in mediating the formation and trafficking of platelet-neutrophil aggregates to the brain, impairing cerebral blood flow and thereby aggravating ischemic stroke outcomes.[6] Importantly, targeting platelet Glycoprotein VI, a key mediator of procoagulant platelet formation, protects hyperglycemic mice from ischemic stroke, indicating a crucial role for procoagulant platelets also in the setting of hyperglycemic stroke.[7]
Platelet glucose is a key regulator of platelet activation and the formation of procoagulant platelets.[8] In addition, platelets in the setting of hyperglycemia, demonstrate increased activation and contribute to in vivo thrombosis, which can be prevented when platelet glucose metabolism is abolish through deletion of Glut1 and Glut3, the primary glucose transporters in platelets.[9] However, the role of platelet glucose metabolism in ischemic stroke injury has never been examined. Furthermore, whether procoagulant platelet formation in the setting of hyperglycemia regulates ischemia-reperfusion injury is completely unknown. In this study, we investigated the role of platelet glucose metabolism in ischemic stroke under normoglycemic and hyperglycemic conditions. While platelet glucose metabolism regulates the formation of procoagulant platelet formation and ischemic stroke brain injury during normoglycemia, we surprisingly found hyperglycemia potentiates procoagulant platelet formation and exacerbates ischemic stroke brain injury independent of platelet glucose uptake.
Materials and Methods
Mice
All animal experiments complied with the regulatory standards of the University of Utah and were performed following the ARRIVE guidelines (www.nc3rs.org.uk), including randomization and analysis blind to the genotype. The generation of platelet specific Glut1 and Glut3 deficient mice has been previously described.[8] All experiments were performed using 8- to 12-week-old age- and gender-matched littermates. Male and female mice were used in all experiments.
Streptozotocin induced hyperglycemia
Streptozotocin (STZ) (Sigma Aldrich, St. Louis, MO) was dissolved in 0.1 M sodium citrate and administered to 4 hours fasted mice by intraperitoneal injections at 50 mg/kg daily for 5 consecutive days at 6 weeks of age. Mice were housed for an additional two weeks before using them in experiments. Non-fasting blood glucose concentrations were determined by tail-clip blood draw with a Contour clinical glucometer (Bayer, Leverkusen, Germany). Only mice with non-fasting blood glucose exceeding 300 mg/dL were used for experiments.
Ischemic stroke model
Transient middle cerebral artery occlusion (tMCAO) was performed as described previously.[6] Briefly, occlusion of the right MCA was achieved by inserting a standardized monofilament (Doccol Corp, Redlands, CA) via the right internal carotid artery to occlude the origin of the right MCA. The occluding suture was left in situ for 60 minutes. Induction of ischemic stroke was confirmed by neurological testing of the mice while the MCA was occluded. Anesthesia was induced by inhalation of 5% isoflurane and maintained by inhalation of 2% isoflurane. Buprenorphine was administered one hour before surgery and every 12 hours as needed. Sham surgery was performed similarly, without insertion of the monofilament.
The following conditions excluded mice from endpoint analyses (exclusion criteria): (1) death within 24 hours after tMCAO, (2) operation time > 10 minutes or (3) when surgical complications occurred. Of the 120 mice subjected to tMCAO, 17 mice (14.1%) met at least 1 of the exclusion criteria after randomization. Five were excluded because they died during the time of the experiment (exclusion criterion (1)). No difference in mortality rate was observed between the groups assessed. The remaining 12 mice were excluded due to surgical complications determined after sacrifice (exclusion criterion (3)). During these experiments, no mice needed to be eliminated due to exclusion criterion (2): operation time >10 minutes.
Neurological Tests
Twenty-four hours after ischemic stroke, neurological and motor functions were assessed as described previously by the modified Bederson test and the grip test, respectively.[6]
Cerebral lesion quantification
To quantity ischemic stroke brain damage, 2-mm-thick coronal brain sections were stained with 2% 2,3,5-triphenyl-tetrazolium chloride (TTC, Sigma-Aldrich, Saint Louis, MO) to distinguish unaffected brain tissue from infarcted tissue, 24 hours after stroke induction. Stained slices were photographed and infarct areas (white) were measured using Image J software (National Institutes of Health, Bethesda, MD) by an operator blinded for genotype.
Evans blue extravasation
Twenty-four hours after stroke, mice were injected intravenously via the retroorbital plexus with 100 μL 2% Evans Blue. Two hours later, mice were euthanized and perfused with phosphate buffered saline. To quantify Evans blue extravasation, 2-mm-thick coronal sections were photographed and measured using Image J software (National Institutes of Health, Bethesda, MD) by an operator blinded for genotype.
Monitoring of cerebral blood flow
For cerebral blood flow measurements, a fiber optic probe (moorVMS-LDF1; Moor Instruments; Devon, UK) was placed in the vascular territory of the right MCA. Changes in blood flow were determined by laser Doppler flow.[10]
Analysis of whole blood platelet activation
Twenty-four hours after stroke, blood was collected and diluted 1:100 into M199 supplemented with 100 U/mL heparin. Diluted whole blood was stained with rat anti-mouse CD41 APC (MWREG30, ThermoFisher), anti-P-selectin (Wug.E9-PE, Emfret) and JON/A antibody (M023-2, Emfret). Samples were incubated for 15 minutes at 37°C and subsequently fixed with BD FACS lysis buffer and analysed on a Beckman Coulter Cytoflex (Beckman-Coulter, Pasadena, CA) located in the Utah Flow Cytometry Core.
Analysis of circulating Platelet-Leukocyte Aggregates
Twenty-four hours after stroke, blood was collected and diluted 1:10 into M199 supplemented with 100 U/mL heparin. For the detection of platelet-neutrophil aggregates, diluted blood was stained with rat anti-mouse CD41 APC (MWREG30, ThermoFisher) and rat anti-mouse Ly6G BV510 (Biolegend, San Diego, CA). For the detection of platelet-monocyte aggregates, diluted blood was stained with rat anti-mouse CD41 APC (MWREG30, ThermoFisher) and rat anti-mouse Ly6C FITC. Samples were incubated for 15 minutes at 37°C, fixed with BD FACS lysis buffer and analysed on a Beckman Coulter Cytoflex located in the Utah Flow Cytometry Core.
Analysis of circulating procoagulant platelets
Twenty-four hours after stroke, mice were euthanized, and platelets were isolated from whole blood after cardiac puncture. Whole blood was drawn into sodium citrate (1:9), diluted to 2ml with PIPES-saline-glucose buffer (PSG) and centrifuged at 150 x g for 5 minutes. PGE1 was added to the platelet-rich plasma which was subsequently centrifuged at 400xg for 10min. Platelets were resuspended in PSG and subsequently diluted to a concentration of 1x 107 in M199. Washed platelets were stained with rat anti-mouse CD41 APC (MWREG30) and FITC-conjugated annexinV (BioLegend) for 15 minutes and immediately analysed on a Beckman Coulter Cytoflex located in the Utah Flow Cytometry Core.
Flow cytometric analysis of cerebral neutrophil recruitment
Twenty-four hours after stroke, mice were euthanized and single-cell suspensions were made of the ischemic (ipsilateral) hemispheres as previously described.[11] Cells were incubated with CD45 APC-cy7 (Biolegend), CD11b PE-cy7 (Ebioscience), Ly6G BV510 (Biolegend), CD41 APC (MWREG30, ThermoFisher) and CD16/CD32 (Fc-block, EBioscience) for 30 minutes at room temperature in PBS + 5% FBS. Thirty minutes later, cells were washed, fixed and ran on a Beckman Coulter Cytoflex located in the Utah Flow Cytometry Core.
Procoagulant platelet formation assay
Blood was drawn from the retro-orbital plexus into sodium citrate (1:9) and diluted 1:1 with PIPES-Saline-Glucose (PSG). Platelets were washed twice in PSG before being resuspended at a concentration of 1x 107 in M199. Washed platelets were stained with rat anti-mouse CD41 APC (MWREG30) and FITC-conjugated annexinV (BioLegend) and stimulated for 15 minutes with 150 ng/mL of CRP and 0.05 U/mL of thrombin. Samples were immediately analysed on a Beckman Coulter Cytoflex located in the Utah Flow Cytometry Core.
Mitochondrial ROS measurement
To measure mitochondrial reactive oxygen species, washed platelets were resuspended in M199 in the presence of MitoTracker Green (200 nM, final; ThermoFisher) and MitoSox Red (200 nM, final; ThermoFisher) for 40 minutes at 37°C. Samples were immediately run on a Beckman Coulter Cytoflex.
Western blotting
Platelets were isolated and analyzed for protein after cardiac puncture as described before.[12]
Statistical analyses
For statistical analyses Graph Pad Prism Version 8.1.1. was used (GraphPad Software, La Jolla, CA). Normality of data was done prior to statistical analysis by a D’Agostino and Pearson normality test. The number of animals included in each group was based on power calculations with infarct volume as primary parameter and with mean differences and standard deviations taken from available data from the same tMCAO model (power of 80% and α of 0.05).
An unpaired T-test or one-way ANOVA with Dunnett’s post-hoc test or a Mann-Whitney test was used for statistical comparison when appropriate. In the case of non-parametric data (Bederson and grip-test score) a Kruskal–Wallis test with post-hoc Dunn correction was performed. A repeated measures ANOVA was used to compare changes in laser Doppler blood flow. All data are represented as mean ± standard deviation. A 2-tailed P < 0.05 was considered statistically significant.
Results
Ablation of platelet glucose metabolism protects mice from ischemic stroke brain injury.
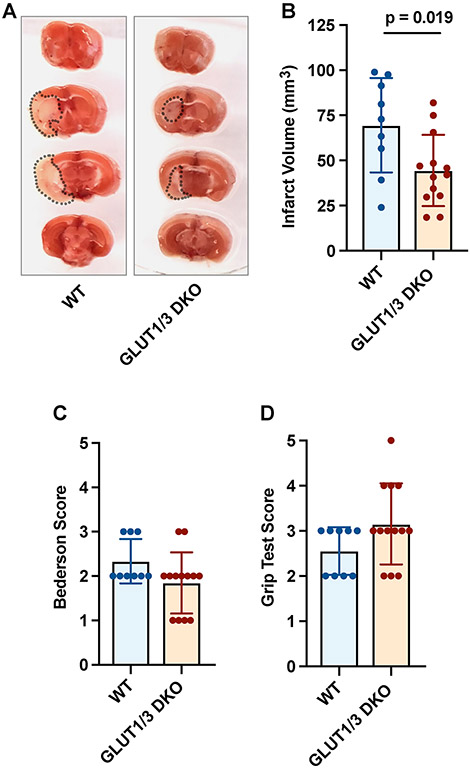
Platelet-specific Glut1 and Glut3 double knock-out (DKO) mice are unable to take up glucose from the extracellular compartment and can therefore not metabolize glucose in their platelets.[8] To examine the contribution of platelet glucose metabolism to ischemic stroke, DKO mice and their littermate wild-type (WT) controls were subjected to one hour tMCAO. Ischemic stroke brain damage was determined 24 hours after stroke onset by measuring infarct size. Infarct volumes were significantly smaller in mice lacking platelet Glut1 and Glut3 (Figure 1A-B). On average, WT mice had infarct volumes of 69.5 ± 26.1 mm3 while average infarct volumes in DKO mice were 44.5 ± 19.8mm3 (p = 0.019). The reduction in infarct size was associated minor improvement in neurological scoring (measured with the Bederson Test, Figure 1C) and motor function (measured with the Grip Test, Figure 1D).
Figure 1. Platelet-specific deficiency of Glut 1 and 3 protects mice from acute ischemic stroke.
Platelet-specific Glut 1 and 3 deficient (DKO) mice or control mice (WT) were subjected to one-hour tMCAO. (A) Representative brain sections were stained with 2,3,5-triphenyl-tetrazolium chloride. Red areas indicate healthy brain tissue, while white areas show infarcted brain tissue. (B) Quantification of brain infarct volumes 24 hours after stroke. (C) The Bederson test was used to assess neurological outcome 24 hours after stroke. A lower score indicates better neurologic function. (D) To measure motor function, we employed a grip test 24 hours after stroke. A higher score indicates better motor function.
Platelet glucose metabolism is required for thrombo-inflammation after stroke.
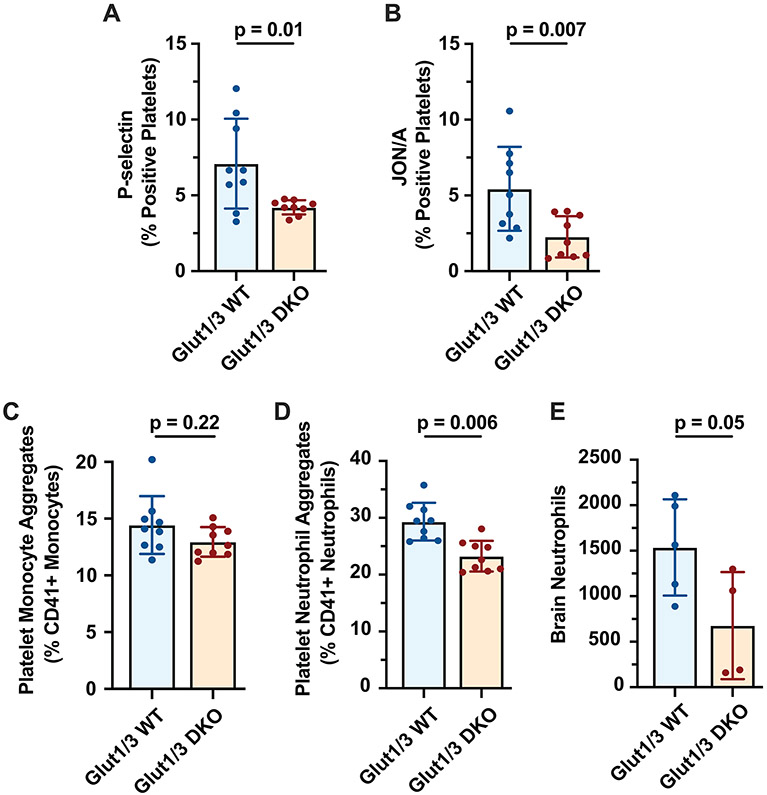
Previously, it was shown that DKO mice have impaired platelet activation responses.[8] To investigate whether stroke-induced platelet activation was different between DKO and WT mice, we measured platelet P-selectin expression and JON/A binding, a marker for GPIIbIIIa activation. Platelets from DKO mice expressed significantly less platelet P-selectin (Figure 2A) and had significantly less JON/A binding (Figure 2B) compared to WT mice 24 hours after stroke. As P-selectin and GPIIbIIIa are important receptors for platelet-leukocyte aggregate formation, we subsequently measured platelet-neutrophil and platelet-monocyte aggregates 24 hours after stroke. Circulating platelet-neutrophil aggregates (PNAs) were significantly increased in WT mice compared to DKO mice (29.3 ± 3.3% vs. 23.3 ± 2.7%, p=0.0006; Figure 2D). In contrast, circulating platelet-monocyte aggregates (PMAs) were not different (Figure 2C).
Figure 2. Platelet glucose metabolism mediates thromboinflammation during ischemic stroke brain injury.
Platelet-specific Glut 1 and 3 deficient (DKO) mice or control mice (WT) were subjected to one-hour tMCAO. Twenty-four hours after stroke onset, blood was drawn and expression of platelet P-selectin (A), binding of JON/A (B), (C) circulating platelet-monocyte aggregates (CD41+ Ly6G− Ly6C+ cells) and circulating platelet-neutrophil aggregates (CD41+ Ly6G+ cells) (D) were quantified in whole blood as described in the methods. (E) Recruitment of neutrophils to the brain was quantified 24 hours after stroke as described in the methods.
As neutrophils are critical drivers of ischemic stroke brain injury, we next examined cerebral neutrophil recruitment in WT and DKO mice. The infarcted brain hemisphere from WT and DKO mice was dissected 24 hours after stroke, stained for neutrophils, and analyzed by flow cytometry. Quantification of neutrophil recruitment demonstrated a 50% decrease in neutrophil recruitment to the affected brain hemispheres of DKO mice compared to WT mice (1537±529 vs. 678±588, p=0.05; Figure 2E).
Hyperglycemia worsens ischemic stroke outcome irrespective of platelet glucose uptake
To examine the direct consequence of altered glucose utilization in platelets in the setting of hyperglycemic stroke, we induced type 1 diabetes (T1D) in DKO mice and littermate WT controls. The day before surgery, blood glucose was significantly elevated in T1D WT and DKO mice compared to vehicle-treated controls (465.1 ± 66.4 vs. 132.9 ± 24.1 mg/dL and 465.3 ± 99.8 vs. 137.3 ± 19 mg/dL, respectively; p<0.0001; Supplemental Figure 1A). Previously, we have demonstrated platelet glucose metabolism and glycogen content are altered only in platelets from WT mice not DKO mice under normo- and hyperglycemic conditions.[9] Here we found blood glucose levels evolved similarly during ischemia and after reperfusion in WT and DKO mice under normoglycemic and hyperglycemic conditions (Supplemental Figure 1B).
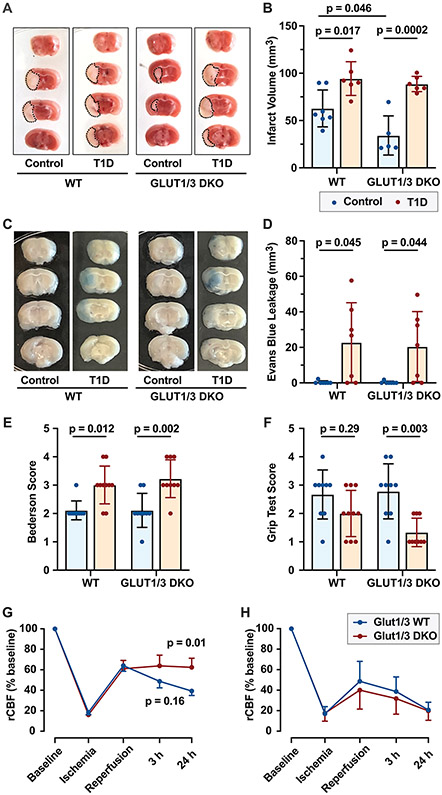
The effect of hyperglycemia on cerebral ischemia/reperfusion injury was determined by comparing ischemic stroke brain damage and stroke outcomes 24 hours after tMCAO in T1D and control WT and DKO mice. Hyperglycemia significantly increased infarct volume in WT mice (Figure 3A and B). Unexpectedly, hyperglycemia induced an even more pronounced increase in infarct volume in DKO mice (Figure 3A and B). As hyperglycemia induces blood brain barrier (BBB) dysfunction, we next examined Evans blue extravasation after stroke in WT and DKO mice under normoglycemic and hyperglycemic conditions. In normoglycemic mice, BBB disruption was not observed either in WT or DKO mice (Figure 3C and D). However, hyperglycemia induced significant BBB disruption to a similar extent in WT and DKO mice, 24 hours after stroke (Figure 3C and D). Accordingly, hyperglycemia was associated with worsened neurological and motor outcome in WT and DKO mice (Figure 3E and F).
Figure 3: Hyperglycemia exacerbates ischemic stroke outcomes independent of platelet glucose uptake.
Platelet-specific Glut 1 and 3 deficient (DKO) mice or control mice (WT) were subjected to one-hour tMCAO under normoglycemic or STZ-treated hyperglycemic conditions. (A) Representative brain sections were stained with 2,3,5-triphenyl-tetrazolium chloride. Red areas indicate healthy brain tissue, while white areas show infarcted brain tissue. (B) Quantification of brain infarct volumes 24 hours after stroke. (C-D) WT and DKO mice were injected with Evans blue 24 hours after stroke, two hours later mice were perfused, and Evans blue leakage into the brain was examined. Representative brain sections demonstrating disruption of the blood brain barrier (C). (D) Evans blue extravasation was quantified as described in the methods. (E) The Bederson test was used to assess neurological outcome 24 hours after stroke. A lower score indicates better neurologic function. (F) To measure motor function, we employed a grip test 24 hours after stroke. A higher score indicates better motor function. (G-H) CBF was examined in the right MCA territory before stroke (baseline), during ischemia, when reperfusion was allowed and at 3 and 24 hours after stroke induction under normoglycemic (G) and hyperglycemic conditions (H). (N = 5 per group).
Finally, we examined cerebral blood flow (CBF) in WT and DKO mice under normo- and hyperglycemic conditions to determine if stroke outcomes could be attributed to differences in CBF. Under normoglycemic conditions, DKO mice had greater CBF in the reperfusion phase after stroke (Figure 3G). During ischemia, and when reperfusion was allowed, CBF was undistinguishable between WT and DKO mice. However, 3 hours after stroke onset, CBF was improved in DKO mice compared to WT mice (63.8 ± 6.4% vs. 48.7 ± 6.4%, p = 0.16). This change in CBF continued and became significant at 24 hours (62.4 ± 8.9% vs. 39.3 ± 4.3%, p = 0.01). Under hyperglycemic conditions, CBF was similarly impaired in WT and DKO mice at all time points assessed in the reperfusion phase (Figure 3H). Interestingly, CBF dropped faster and more severely in hyperglycemic mice compared to normoglycemic mice in both WT and DKO animals (Figure 3G and H).
Platelet-specific Glut1 and Glut3 deficiency does not affect thrombo-inflammation under hyperglycemic conditions
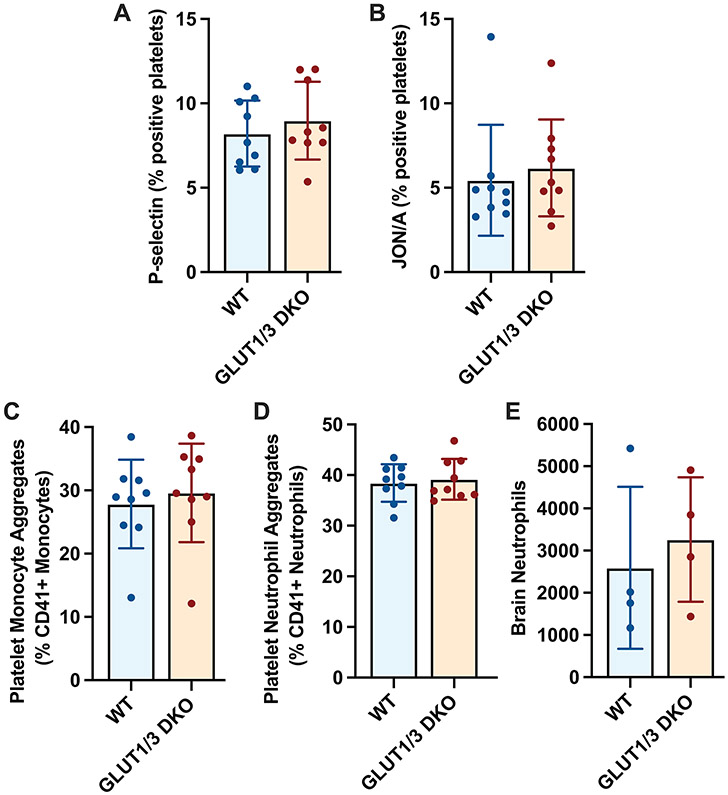
Since platelet activation and neutrophil trafficking to the brain were significantly different between WT and DKO mice after stroke under normoglycemic conditions, we wanted to investigate whether this was still different under hyperglycemic conditions. To address this, we examined platelet activation, platelet-leukocyte aggregate formation and cerebral neutrophil recruitment in the setting of stroke under hyperglycemic conditions using flow cytometry. Both platelet P-selectin expression and JON/A binding were similar between hyperglycemic WT and DKO mice, 24 hours after stroke (Figure 4A and B). Likewise, circulating platelet-neutrophil aggregates and platelet-monocyte aggregates were similar between hyperglycemic WT and DKO mice (Figure 4C and D). This resulted in similar neutrophil trafficking to the brain in hyperglycemic WT and DKO mice (Figure 4E). Interestingly, in particular platelet-leukocyte aggregate formation was increased when hyperglycemic mice were compared to normoglycemic mice (Figure 2 and 4). Taken together, our results demonstrate hyperglycemia increases the formation of platelet-neutrophil complexes and the recruitment of neutrophils to the ischemic stroke brain, independent of platelet glucose uptake.
Figure 4: Thrombo-inflammation is similar between WT and DKO hyperglycemic mice.
Platelet-specific Glut 1 and 3 deficient (DKO) mice or control mice (WT) were subjected to one-hour tMCAO under STZ-treated hyperglycemic conditions. Twenty-four hours after stroke onset, blood was drawn and expression of platelet P-selectin (A), binding of JON/A (B), circulating platelet-neutrophil aggregates (CD41+ Ly6G+ cells) (C) and circulating platelet-monocyte aggregates (CD41+ Ly6G− Ly6C+ cells) (D) were quantified in whole blood as described in the methods. (E) Recruitment of neutrophils to the brain was quantified as described in the methods 24 hours after stroke.
Hyperglycemia potentiates procoagulant platelet formation during ischemic stroke
Recently, we discovered procoagulant platelets as critical mediators of detrimental platelet-neutrophil interactions during cerebral ischemia/reperfusion injury.[6] Procoagulant platelets are a distinct population of activated platelets expressing high levels of phosphatidyl serine on their surface (PShigh). To investigate the contribution of procoagulant platelets to hyperglycemia mediated exacerbation of stroke, we measured circulating procoagulant platelets in WT and DKO mice subjected to sham surgery or stroke under normoglycemic and hyperglycemic conditions 24 hours after surgery (Figure 5A, Supplemental Figure 2). Stroke induced a significant increase in circulating procoagulant platelets in WT animals (4.7 ± 0.8% vs. 0.7 ± 0.18%, p = 0.001). In contrast, procoagulant platelets were not increased in DKO animals after stroke (2.7 ± 0.8% vs. 1.3 ± 0.3%, p = 0.4). Moreover, hyperglycemia increased the amount of procoagulant platelets circulating after stroke both in WT (7 ± 1.9%; p = 0.02) and DKO mice (6.2 ± 1.8%; p = 0.0004). Of note, circulating procoagulant platelets were unaffected by hyperglycemia in sham operated animals.
Figure 5: Hyperglycemia potentiates procoagulant platelet formation.
(A) Platelet-specific Glut 1 and 3 deficient (DKO) mice or control mice (WT) were subjected to one-hour tMCAO or sham surgery under normoglycemic or STZ-treated hyperglycemic conditions. Phosphatidylserine (PS) exposure was assessed by flow cytometry using Annexin V binding. The percentage of platelets with high PS exposure were quantified. (B) Platelets were isolated from normoglycemic or STZ-treated hyperglycemic DKO and WT mice in the absence of surgery. PS exposure was assessed by flow cytometry using Annexin V binding at baseline and after dual stimulation with thrombin and CRP. The percentage of platelets with high PS exposure were quantified.
To investigate if the observed increase in procoagulant platelets was due to increased stroke severity or whether platelets from hyperglycemic mice had an increased procoagulant platelet potential, we isolated platelets from healthy and T1D WT and DKO mice and studied their ability to make procoagulant platelets in vitro. Interestingly, when healthy DKO platelets were stimulated with thrombin and collagen related peptide (CRP), only 2.2±0.5% platelets expressed high levels of PS on their surface, while in healthy WT animals this was 11.3±3.1% of platelets (Figure 5B, Supplemental Figure 3). Conversely, hyperglycemia mildly potentiated procoagulant platelet formation in WT animals (16.4±3% PShigh platelets, p=0.042) and restored procoagulant platelet formation in DKO animals (11.3±4.3% PShigh platelets, p=0.0003).
Hyperglycemia induces mitochondrial dysfunction independent of platelet glucose uptake
In the same model of streptozotocin-induced hyperglycemia, it was previously shown that Cyclophilin D (CypD) expression was increased in brain mitochondria.[13] As CypD is essential in the formation of procoagulant platelets, we hypothesized increased expression of CypD could potentiate procoagulant platelet formation. However, no increase in CypD expression was observed in platelets isolated from hyperglycemic mice (Supplemental Figure 4).
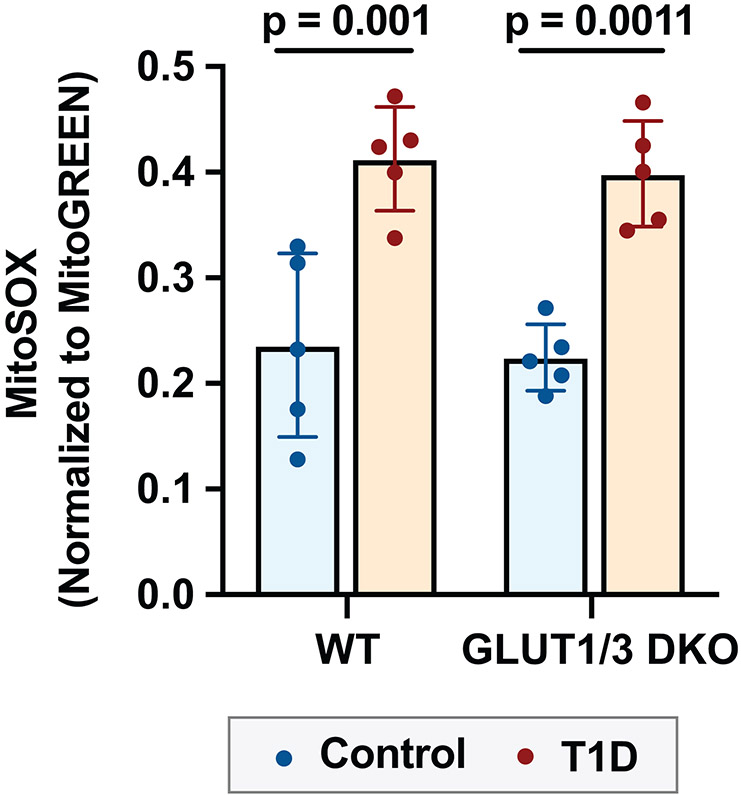
Procoagulant platelet formation is dependent on platelet mitochondria and mitochondrial dysfunction could contribute to procoagulant platelet formation through increased generation of reactive oxygen species (ROS). To address this, we measured mitochondrial ROS generation by MitoSOX staining in WT and DKO mice, under normoglycemic and hyperglycemic conditions. Hyperglycemia increased mitochondrial ROS production both in WT and DKO mice (Figure 6).
Figure 6: Hyperglycemia increases mitochondrial ROS in platelets independent of platelet glucose uptake.
Platelets were isolated from platelet specific Glut 1 and 3 deficient (DKO) mice or littermate controls (WT). Mitochondrial ROS was measured by MitoSOX staining normalized to MitoGreen, analyzed by flow cytometry under resting conditions.
Discussion
In this study, we demonstrated that platelet glucose metabolism is essential for platelet activation and platelet-leukocyte aggregate formation contributing to ischemic stroke brain injury in healthy adult mice. Surprisingly, this protective phenotype was eliminated in hyperglycemic, diabetic mice. These results imply platelet dysfunction caused by systemic hyperglycemia rather than direct platelet glucose uptake contributes to exacerbated stroke outcomes in diabetic mice.
Previously, we demonstrated that simultaneous deletion of platelet glucose transporters GLUT1 and GLUT3 completely abolishes platelet glucose uptake.[8] This lack of glucose metabolism results in decreased platelet activation and thrombosis through impairment of Ca2+ signaling, degranulation, and integrin activation. In line with these previous results, we now report similar observations in the setting of ischemic stroke. After ischemic stroke brain injury, we observed less α-granule release, as measured by platelet P-selectin expression, and reduced activation of the integrin GPIIbIIIa. Furthermore, platelet-neutrophil aggregate formation was reduced in GLUT1/3 DKO mice. This reduction in platelet activation and subsequent platelet-neutrophil aggregate formation resulted in reduced cerebral inflammation and enhanced cerebral blood flow, contributing to improved ischemic stroke outcomes. While deletion of GLUT1/3, significantly reduced infarct size, we observed little improvement in neurological and motor function potentially due to modest reduction in infarct size.[14] Of note, GLUT1/3 DKO mice also have reduced platelet counts due to impaired pro-platelet formation and increased platelet clearance.[8] However, since only severe thrombocytopenia protects mice from ischemic stroke brain injury[15], the ~40% reduction in platelet counts in GLUT1/3 DKO mice is unlikely to contribute to the observed protective effect in our stroke model.
Patients with hyperglycemia are at higher risk of ischemic events[1,2,16-18] and this is partially attributed to increased platelet activation.[19] Importantly, inhibition of platelet receptors GPIb or GPVI protect hyperglycemic mice from ischemic stroke, underscoring the role of platelets in ischemic stroke brain injury under hyperglycemic conditions.[7] Previous studies have correlated platelet activation with glucose metabolism[20] and in a model of pulmonary embolism, the hyperglycemia-associated increase in thrombosis could be abolished by platelet-specific deletion of GLUT1 and 3.[9] In contrast, we now report that platelet glucose metabolism is not involved in hyperglycemia-aggravated ischemic stroke outcomes. Our results highlight the complexity of ischemic stroke pathophysiology and offer a potential explanation for the failure of clinical trials acutely lowering glucose levels in stroke patients.[21] Independent of platelet glucose uptake, hyperglycemia increased platelet activation and procoagulant platelet formation after stroke and potentiated platelet-neutrophil interactions resulting in aggravated ischemic stroke brain injury, disruption of the blood brain barrier, and worsened neurological and motor outcomes.
Platelets and neutrophils cooperate to exacerbate ischemic stroke brain injury[3], and this is more pronounced under hyperglycemic conditions.[4] We recently found a critical role for procoagulant platelets mediating the formation of detrimental platelet-neutrophil aggregates in ischemic stroke.[6] Interestingly, platelets from DKO mice are unable to form procoagulant platelets[8], which may contribute to the protective phenotype of DKO mice in this stroke model. Conversely, we found hyperglycemic DKO mice can form procoagulant platelets. We also observed hyperglycemic WT mice generated more procoagulant platelets. Previously, it was demonstrated that diabetes potentiates GPVI-mediated platelet activation[20,22] and induces mitochondrial dysfunction leading to the formation of procoagulant platelets.[23] Our observation of increased procoagulant activity irrespective of platelet glucose uptake imply glucose uptake is not the sole driver of platelet hyperreactivity in diabetes. Besides glucose, free fatty acids[9], oxLDL[24,25] and cytokines such as TNFα[26,27] are increased in diabetes. Importantly, all of these have been shown to increase platelet reactivity and even procoagulant platelet formation, due to mitochondrial ROS production.[28-30] In our study, we report increased mitochondrial ROS in both hyperglycemic WT and DKO mice, implying systemic hyperglycemia rather than direct glucose uptake by platelets contributes to mitochondrial disfunction in platelets from diabetic mice. Furthermore, DKO platelets under metabolic stress have increased mitochondrial dysfunction, resulting in increased calpain activation and phosphatidylserine exposure[8], which might also contribute to increased procoagulant platelet formation in DKO platelets. An interesting player in all of these processes is the scavenging receptor CD36 and its downstream effector ERK5, which is a redox-sensor.[31] Signaling through this pathway by various agents has been shown to potentiate GPVI mediated signaling and subsequent procoagulant platelet formation.[31] We hypothesize the increase in procoagulant platelets drives the increase in injurious platelet-neutrophil interactions in the hyperglycemic stroke brain. However, future research is warranted to investigate how procoagulant platelets formation is altered in the setting of diabetes and stroke and whether targeting procoagulant platelet formation confers protection from ischemic stroke brain injury under hyperglycemic conditions.
Ischemia reperfusion injury is common in other critically ill-patients, including cardiac[32], gut[33] and renal ischemia[34] where thrombosis is common. While our findings are mostly directly applicable to cerebral ischemia, our results may have significant implications to these other disease states where diabetes also significantly contributes to morbidity.[35,36] In our study, we also observed increased circulating platelet-monocyte aggregates after stroke in hyperglycemic mice. While previous studies have found no effect for monocytes in models of acute ischemic stroke[37], the increase in platelet-monocyte interactions under hyperglycemic conditions might contribute to other thromboinflammatory diseases. Lastly, a limitation of our study is we primarily focused on platelet glucose metabolism. While platelets are key players in ischemic stroke brain injury, they are not the only cells that play a role in stroke pathophysiology. Previous studies have shown that hyperglycemia primes neutrophils[38] and activates the endothelium.[39] How glucose metabolism specifically in these cells impacts stroke outcomes remains to be investigated.
In conclusion, we demonstrate hyperglycemia primes procoagulant platelet formation and exacerbates ischemic stroke outcomes independent of platelet glucose uptake. This underlines the therapeutic potential for strategies targeting procoagulant platelet formation for the treatment of ischemic stroke.
Supplementary Material
Essentials.
Hyperglycemia is a comorbidity for ischemic stroke and mediates platelet hyperactivity.
Dysregulated platelet glucose metabolism protects mice from ischemic stroke under normoglycemic conditions.
Hyperglycemia potentiates procoagulant platelet formation and aggravates ischemic stroke outcomes independent of platelet glucose uptake.
Hyperglycemia increases mitochondrial ROS in platelets independent of platelet glucose uptake.
Acknowledgements
We thank Ms. Diana Lim for her assistance with creating the figures.
Sources of Funding
This work was supported by the NHLBI and NIA (K01AG059892, U10NS086606 to R.A.C) and a Utah Stroke Net Fellowship 4U10NS086606 to F.D.. F.D. is a postdoctoral fellow of the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (FWO, 12U7818N).
Footnotes
Disclosures
The authors have nothing to disclose.
References
- 1.Williams LS, Rotich J, Qi R, Fineberg N, Espay A, Bruno A, Fineberg SE, Tierney WR. Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology 2002; 59: 67–71. [DOI] [PubMed] [Google Scholar]
- 2.Desilles J-P, Meseguer E, Labreuche J, Lapergue B, Sirimarco G, Gonzalez-Valcarcel J, Lavallée P, Cabrejo L, Guidoux C, Klein I, Amarenco P, Mazighi M. Diabetes Mellitus, Admission Glucose, and Outcomes After Stroke Thrombolysis. Stroke 2013; 44: 1915–23. [DOI] [PubMed] [Google Scholar]
- 3.De Meyer SF, Denorme F, Langhauser F, Geuss E, Fluri F, Kleinschnitz C. Thromboinflammation in Stroke Brain Damage. Stroke 2016; 47: 1165–72. [DOI] [PubMed] [Google Scholar]
- 4.Desilles J-P, Syvannarath V, Ollivier V, Journé C, Delbosc S, Ducroux C, Boisseau W, Louedec L, Di Meglio L, Loyau S, Jandrot-Perrus M, Potier L, Michel J-B, Mazighi M, Ho-Tin-Noé B. Exacerbation of Thromboinflammation by Hyperglycemia Precipitates Cerebral Infarct Growth and Hemorrhagic Transformation. Stroke 2017; 48: 1932–40. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Chopp M, Zhang Y, Xiong Y, Li C, Sadry N, Rhaleb I, Lu M, Zhang ZG. Diabetes Mellitus Impairs Cognitive Function in Middle-Aged Rats and Neurological Recovery in Middle-Aged Rats After Stroke Stroke Lippincott Williams & Wilkins; 2016; : STROKEAHA.115.012578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denorme F, Manne BK, Portier I, Eustes AS, Kosaka Y, Kile BT, Rondina MT, Campbell RA. Platelet necrosis mediates ischemic stroke outcome in mice. Blood 2020; 135: 429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraft P, Schuhmann MK, Fluri F, Lorenz K, Zernecke A, Stoll G, Nieswandt B, Kleinschnitz C. Efficacy and Safety of Platelet Glycoprotein Receptor Blockade in Aged and Comorbid Mice With Acute Experimental Stroke Stroke Lippincott Williams & Wilkins; 2015; 46: 3502–6. [DOI] [PubMed] [Google Scholar]
- 8.Fidler TP, Campbell RA, Funari T, Dunne N, Angeles EB, Middleton EA, Chaudhuri D, Weyrich AS, Abel ED. Deletion of GLUT1 and GLUT3 Reveals Multiple Roles for Glucose Metabolism in Platelet and Megakaryocyte Function CellReports ElsevierCompany; 2017; 20: 881–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fidler TP, Marti A, Gerth K, Middleton EA, Campbell RA, Rondina MT, Weyrich AS, Abel ED. Glucose Metabolism is Required for Platelet Hyperactivation in a Murine Model of Type 1 Diabetes Mellitus Diabetes American Diabetes Association; 2019; 68: db180981–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denorme F, Langhauser F, Desender L, Vandenbulcke A, Rottensteiner H, Plaimauer B, François O, Andersson T, Deckmyn H, Scheiflinger F, Kleinschnitz C, Vanhoorelbeke K, De Meyer SF. ADAMTS13-mediated thrombolysis of t-PA-resistant occlusions in ischemic stroke in mice. Blood 2016; 127: 2337–45. [DOI] [PubMed] [Google Scholar]
- 11.Denorme F, Martinod K, Vandenbulcke A, Denis CV, Lenting PJ, Deckmyn H, Vanhoorelbeke K, De Meyer SF. The von Willebrand Factor A1 domain mediates thromboinflammation, aggravating ischemic stroke outcome in mice. Haematologica 2020; doi: 10.3324/haematol.2019.241042. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manne BK, Münzer P, Badolia R, Walker-Allgaier B, Campbell RA, Middleton E, Weyrich AS, Kunapuli SP, Borst O, Rondina MT. PDK1 governs thromboxane generation and thrombosis in platelets by regulating activation of Raf1 in the MAPK pathway. Journal of Thrombosis and Haemostasis 2018; 16: 1211–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan S, Du F, Wu L, Zhang Z, Zhong C, Yu Q, Wang Y, Lue L-F, Walker DG, Douglas JT, Yan SS. F1F0 ATP Synthase-Cyclophilin D Interaction Contributes to Diabetes-Induced Synaptic Dysfunction and Cognitive Decline Diabetes American Diabetes Association; 2016; 65: 3482–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bieber M, Gronewold J, Scharf A-C, Schuhmann MK, Langhauser F, Hopp S, Mencl S, Geuss E, Leinweber J, Guthmann J, Doeppner TR, Kleinschnitz C, Stoll G, Kraft P, Hermann DM. Validity and Reliability of Neurological Scores in Mice Exposed to Middle Cerebral Artery Occlusion. Stroke 2019; 50: 2875–82. [DOI] [PubMed] [Google Scholar]
- 15.Morowski M, Vogtle T, Kraft P, Kleinschnitz C, Stoll G, Nieswandt B. Only severe thrombocytopenia results in bleeding and defective thrombus formation in mice. Blood 2013; 121: 4938–47. [DOI] [PubMed] [Google Scholar]
- 16.Kraakman MJ, Lee MK, Al-Sharea A, Dragoljevic D, Barrett TJ, Montenont E, Basu D, Heywood S, Kammoun HL, Flynn M, Whillas A, Hanssen NM, Febbraio MA, Westein E, Fisher EA, Chin-Dusting J, Cooper ME, Berger JS, Goldberg IJ, Nagareddy PR, et al. Neutrophil-derived S100 calcium-binding proteins A8/A9 promote reticulated thrombocytosis and atherogenesis in diabetes. J Clin Invest 2017; 127: 2133–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sambola A, Ruiz-Meana M, Barba I, Del Blanco BG, Barrabés JA, Lip GY, Vilardosa Ú, Sansaloni S, Rello P, García-Dorado D. Glycative and oxidative stress are associated with altered thrombus composition in diabetic patients with ST-elevation myocardial infarction. International Journal of Cardiology 2017; 243: 9–14. [DOI] [PubMed] [Google Scholar]
- 18.Chamorro A, Brown S, Amaro S, Hill MD, Muir KW, Dippel DWJ, van Zwam W, Butcher K, Ford GA, Hertog den HM, Mitchell PJ, Demchuk AM, Majoie CBLM, Bracard S, Sibon I, Jadhav AP, Lara-Rodriguez B, van der Lugt A, Osei E, Renú A, et al. Glucose Modifies the Effect of Endovascular Thrombectomy in Patients With Acute Stroke. Stroke 2019; 50: 690–6. [DOI] [PubMed] [Google Scholar]
- 19.Maiocchi S, Alwis I, Wu MCL, Yuan Y, Jackson SP. Thromboinflammatory Functions of Platelets in Ischemia-Reperfusion Injury and Its Dysregulation in Diabetes. Semin Thromb Hemost 2018; 44: 102–13. [DOI] [PubMed] [Google Scholar]
- 20.Yamagishi SI, Edelstein D, Du XL, Brownlee M. Hyperglycemia potentiates collagen-induced platelet activation through mitochondrial superoxide overproduction. Diabetes 2001; 50: 1491–4. [DOI] [PubMed] [Google Scholar]
- 21.Johnston KC, Bruno A, Pauls Q, Hall CE, Barrett KM, Barsan W, Fansler A, Van de Bruinhorst K, Janis S, Durkalski-Mauldin VL, Treatment NE, Investigators ST. Intensive vs Standard Treatment of Hyperglycemia and Functional Outcome in Patients With Acute Ischemic Stroke: The SHINE Randomized Clinical Trial. JAMA 2019; 322: 326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang WH, Stitham J, Gleim S, Di Febbo C, Porreca E, Fava C, Tacconelli S, Capone M, Evangelista V, Levantesi G, Wen L, Martin K, Minuz P, Rade J, Patrignani P, Hwa J. Glucose and collagen regulate human platelet activity through aldose reductase induction of thromboxane. J Clin Invest 2011; 121: 4462–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WH T, J S, Y J, R L, SH L, J Du, G A, S G, G S, K M, J H. Aldose reductase-mediated phosphorylation of p53 leads to mitochondrial dysfunction and damage in diabetic platelets. Circulation Circulation; 2014; 129: 1598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toshima S-I, Hasegawa A, Kurabayashi M, Itabe H, Takano T, Sugano J, Shimamura K, Kimura J, Michishita I, Suzuki T, Nagai R. Circulating Oxidized Low Density Lipoprotein Levels ATVB Lippincott Williams & Wilkins; 2000; 20: 2243–7. [DOI] [PubMed] [Google Scholar]
- 25.Kopprasch S, Pietzsch J, Kuhlisch E, Fuecker K, Temelkova-Kurktschiev T, Hanefeld M, Kühne H, Julius U, Graessler J. In vivo evidence for increased oxidation of circulating LDL in impaired glucose tolerance Diabetes American Diabetes Association; 2002; 51: 3102–6. [DOI] [PubMed] [Google Scholar]
- 26.Zauli G, Toffoli B, di Iasio MG, Celeghini C, Fabris B, Secchiero P. Treatment With Recombinant Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand Alleviates the Severity of Streptozotocin-Induced Diabetes Diabetes American Diabetes Association; 2010; 59: 1261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kain V, Kumar S, Puranik AS, Sitasawad SL. Azelnidipine protects myocardium in hyperglycemia-induced cardiac damage. Cardiovasc Diabetol 2010; 9: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choo H-J, Saafir TB, Mkumba L, Wagner MB, Jobe SM. Mitochondrial Calcium and Reactive Oxygen Species Regulate Agonist-Initiated Platelet Phosphatidylserine Exposure. ATVB 2012; 32: 2946–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davizon-Castillo P, McMahon B, Aguila S, Bark D, Ashworth K, Allawzi A, Campbell RA, Montenont E, Nemkov T, D’Alessandro A, Clendenen N, Shih L, Sanders NA, Higa K, Cox A, Padilla-Romo Z, Hernandez G, Wartchow E, Trahan GD, Nozik-Grayck E, et al. TNF-α–driven inflammation and mitochondrial dysfunction define the platelet hyperreactivity of aging Blood American Society of Hematology; 2019; 134: 727–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang M, Kholmukhamedov A, Schulte ML, Cooley BC, Scoggins NO, Wood JP, Cameron SJ, Morrell CN, Jobe SM, Silverstein RL. Platelet CD36 signaling through ERK5 promotes caspase-dependent procoagulant activity and fibrin deposition in vivo. Blood Advances 2018; 2: 2848–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang M, Silverstein RL. CD36 signaling in vascular redox stress Free Radical Biology and Medicine Elsevier B.V; 2019; 136: 159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ziegler M, Wang X, Peter K. Platelets in cardiac ischaemia/reperfusion injury: a promising therapeutic target. Cardiovascular Research 2019; 115: 1178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan Y, Alwis I, Wu MCL, Kaplan Z, Ashworth K, Bark D, Pham A, McFadyen J, Schoenwaelder SM, Josefsson EC, Kile BT, Jackson SP. Neutrophil macroaggregates promote widespread pulmonary thrombosis after gut ischemia. Science Translational Medicine 2017; 9: eaam5861. [DOI] [PubMed] [Google Scholar]
- 34.Jansen MPB, Emal D, Teske GJD, Dessing MC, Florquin S, Roelofs JJTH. Release of extracellular DNA influences renal ischemia reperfusion injury by platelet activation and formation of neutrophil extracellular traps. Kidney International 2017; 91: 352–64. [DOI] [PubMed] [Google Scholar]
- 35.Muroya Y, He X, Fan L, Wang S, Xu R, Fan F, Roman RJ. Enhanced renal ischemia-reperfusion injury in aging and diabetes. American Journal of Physiology-Renal Physiology 2018; 315: F1843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang D, Hu X, Lee SH, Chen F, Jiang K, Tu Z, Liu Z, Du J, Wang L, Yin C, Liao Y, Shang H, Martin KA, Herzog RI, Young LH, Qian L, Hwa J, Xiang Y. Diabetes Exacerbates Myocardial Ischemia/Reperfusion Injury by Down-Regulation of MicroRNA and Up-Regulation of O-GlcNAcylation. JACC Basic Transl Sci 2018; 3: 350–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt A, Strecker J-K, Hucke S, Bruckmann N-M, Herold M, Mack M, Diederich K, Schäbitz W-R, Wiendl H, Klotz L, Minnerup J. Targeting Different Monocyte/Macrophage Subsets Has No Impact on Outcome in Experimental Stroke Stroke American Heart Association, Inc; 2017; 48: 1061–9. [DOI] [PubMed] [Google Scholar]
- 38.Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, Kahn CR, Wagner DD. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med 2015; 21: 815–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Funk SD, Yurdagul A, Orr AW. Hyperglycemia and endothelial dysfunction in atherosclerosis: lessons from type 1 diabetes. Int J Vasc Med 2012; 2012: 569654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.