Figure 1.
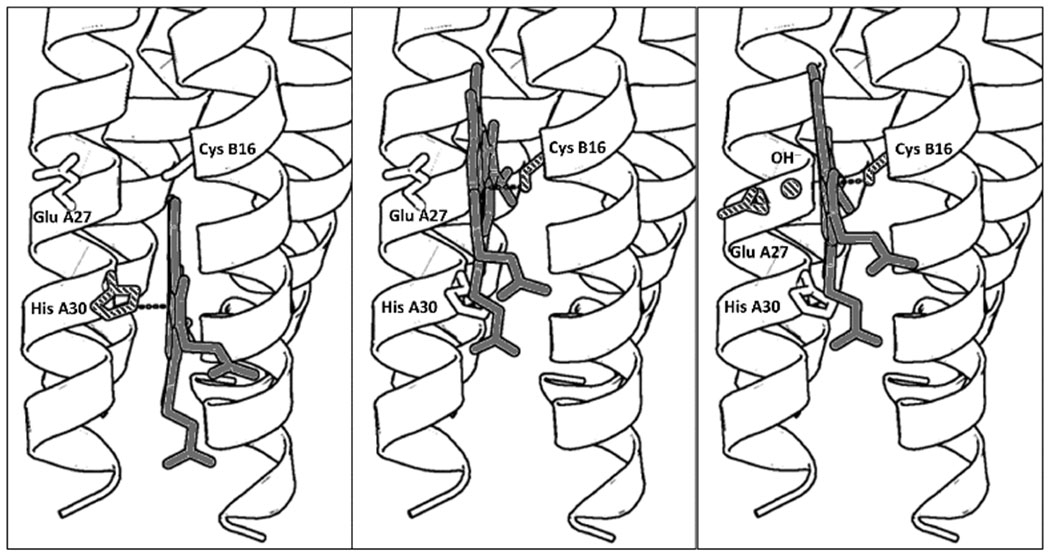
PyMol models illustrating the proposed folding of GRW-L16CL30H peptides complexed with heme (a dimer of antiparallel 2SCC) at selected pH values. The three coordination configurations concluded from our spectroscopic study, i.e. His-pentacoordinated heme (pH 7, left panel), Cys-pentacoordinated heme (pH 9.0, center panel) and Cys/hydroxide hexacoordinated heme (pH 10.5, right panel) are shown. Only the heme for one antiparallel 2SCC (A and B α-helices) is shown for clarity. The PyMol models are based on the crystal structure of a de novo designed antiparallel 4SCC (PDB code: 2B1F) as described in Supplementary Information. A color version of the models is shown in Fig. S5.
