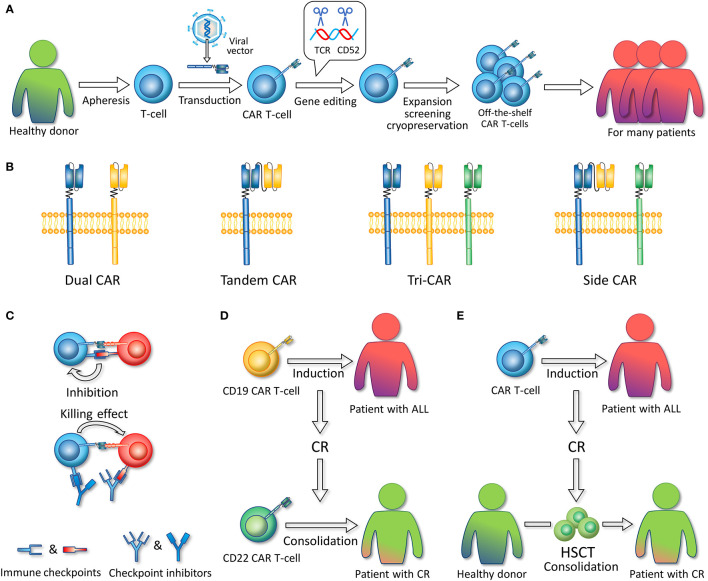
Figure 3.
(A) Allogeneic, off-the-shelf CAR T-cells. The figure illustrates the general preparation process of allogeneic, off-the-shelf CAR T-cells. T-cells are extracted from healthy donors. Viral vectors are used to introduce CAR-encoding genes into T-cells. Gene editing technology is used to remove the gene fragments encoding TCR and CD52. CAR T-cells are expanded, screened, and cryopreserved. These CAR T-cell products can serve as timely treatment for many patients. (B) Multi-antigen targeted CAR. Dual CAR refers to two different mono-CARs in one T-cell. Tandem CAR refers to a CAR structure that contains two single-chain variable fragments. Tri-CAR T-cell coexpresses three different mono-CARs on a single T-cell. Side CAR T-cell expresses a Tandem CAR and a mono-CAR. (C) In combination with immune checkpoint inhibitors. Immune checkpoint inhibitors such as PD-1/PD-L1 inhibitors can specifically block the binding of CAR T-cell or ALL-expressed immune checkpoint molecules to the corresponding receptors on CAR T-cells, thereby enabling CAR T-cell activation and the killing of tumor cells. (D) Sequential infusion. CD19 CAR T-cells are first infused to induce CR, and after patients achieve CR, CD22 CAR T-cells are infused as consolidation. (E) Bridging to HSCT. CAR T-cells are infused to induce CR, and after patients achieve CR, hematopoietic stem cell transplantation (HSCT) is conducted as consolidation.