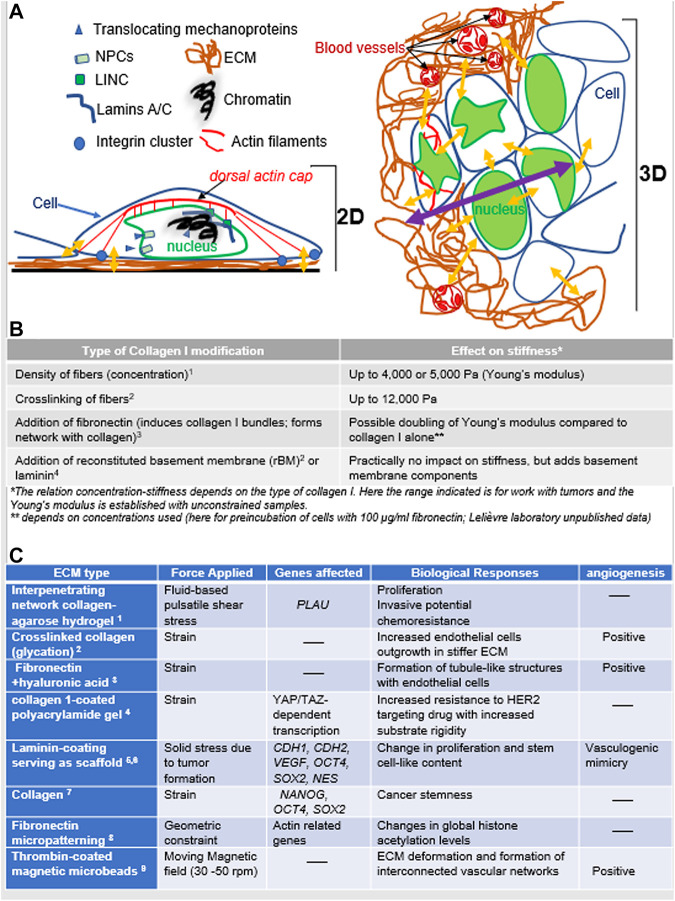
FIGURE 1.
Mechanotransduction in response of mechanostimuli (A). Left: In 2D culture, cancer cells deposit their own ECM on the plastic surface, with focal adhesion (integrin clusters) on the ECM against the hard culture surface; there are also possible tension forces between cells. Two interacting modes of mechanotransduction include 1) the translocation of mechanoproteins (YAP/TAZ, myocardin-related transcription factor; MRTF; muscle LIM protein, MLP, etc.) upon cytoskeletal rearrangement that influence gene transcription and 2) the balance of forces between cytoskeleton and the network of lamins and other nuclear proteins via the LINC complexes, ultimately influencing chromatin compaction and gene transcription. Right: In 3D culture, the organization of cancer cells into a tumor changes not only the type of forces involved but also the intracellular architecture (e.g., different organization of actin microfilaments and no more dorsal actin cap; great variations in nuclear morphometry depending on the epigenome of cells and their location in the tumor). There are variations in the forces received if cells are at the periphery or deep within the tumor. Yellow arrows indicate a sample of areas of mechanostimuli (the impact from the cell culture medium is not included); the purple arrow indicates increasing distances between cells inside the tumor (with multilayering of cells) and the bulk of the ECM that might create heterogeneity in mechanostimuli. Finally, matrix stiffening also increases angiogenesis via an influence on endothelial cells. (B). Examples of ECM stiffness tuning based on collagen I (1 Chhetri et al., 2019, 2 Levental et al., 2009, 3 Paten et al., 2019, 4 Chittiboyina et al., 2018). Other matrices of nonmammalian origin may also be used (e.g., agar, alginate, polyacrylamide) and are sometime mixed with ECM molecules (e.g., collagen I, fibronectin). (C). Cell culture-based examples of the impact of different types of matrices and forces that result in changes in gene transcription and angiogenesis as they relate to cancer aggressiveness (1 Novak et al., 2019, 2 Bordeleau et al., 2017, 3 Seidlits et al., 2011, 4 Lin et al., 2015, 5 Larson et al., 2014, 6 Brodaczewska et al., 2019, 7 Pankova et al., 2019, 8 Jain et al., 2013, 9 Sewell-Loftin et al., 2017).