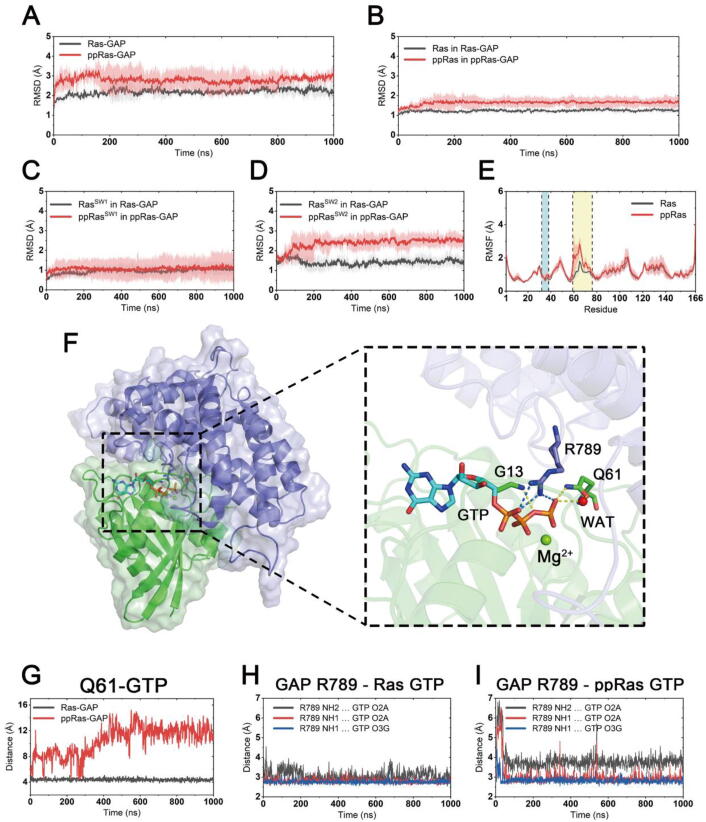
Fig. 5.
Conformational dynamics of unphosphorylated and phosphorylated GTP-bound K-Ras4B in complex with GAP. RMSDs of Cα atoms in unphosphorylated (black) and phosphorylated (red) systems within (A) all Ras and GAP residues; (B) all Ras residues; (C) Ras switch I residues and (D) Ras switch II residues. Gray and pink transparencies represent the error. (E) RMSF of Ras residues. The two switch regions are marked with blue and yellow backgrounds. (F) Representative structure of unphosphorylated K-Ras4B–GAP complex in a GTP-hydrolysis pre-catalytic conformation. (G) Distances from Ras Q61 side chain OE1 atom to GTP Pγ atom. Three atom-pair distances from GAP arginine finger R789 to GTP in the unphosphorylated system (H) and the phosphorylated system (I). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)