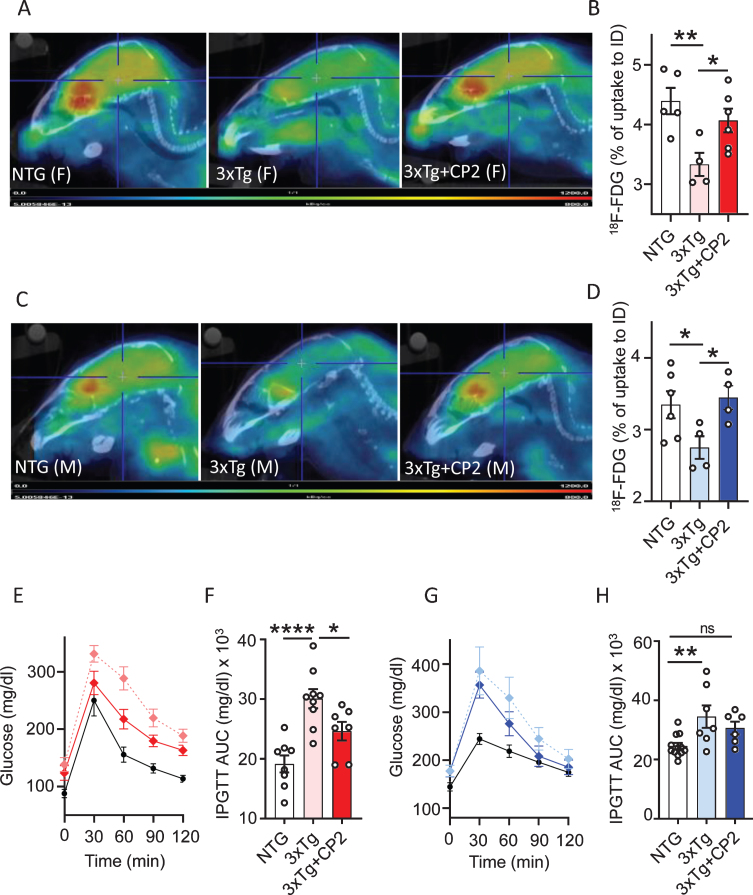
Fig. 2.
Chronic CP2 treatment improves glucose homeostasis in the brain and periphery in 3xTg-AD mice. A,C) Representative images of the 18F-fluorodeoxyglucose (18F-FDG) uptake in the brain of CP2-treated female (A) and male (C) 3xTg-AD mice compared to vehicle-treated age- and sex-matched 3xTg-AD and NTG mice measured using in vivo FDG-PET 14 months after the beginning of treatment. B,D) Quantification of glucose uptake measured using in vivo FDG-PET from (A and C) as a percent of changes compared to the initial dose (ID). N= 5 mice per each group. E,F) CP2 treatment improves glucose tolerance in 3xTg-AD female mice measured by intraperitoneal glucose tolerance test (IPGTT). F) Calculation of Area Under the Curve (AUC) for figure (E). N = 6 –12 mice per group. G,H) CP2 treatment did not significantly improve glucose tolerance in 3xTg-AD male mice. H) Calculation of AUC for figure (G). N= 6–12 mice per group. Differences between individual groups were analyzed by one-way ANOVA with Fisher’s post-hoc test. Data are presented as mean±SEM. *p < 0.05, **p < 0.01, ****p < 0.0001. Dotted light blue and light red: vehicle-treated 3xTg-AD male and female mice, respectfully; dark blue and dark red: 3xTg-AD+CP2 male and female mice, respectfully; black: vehicle-treated NTG (female mice in E; male mice in G).