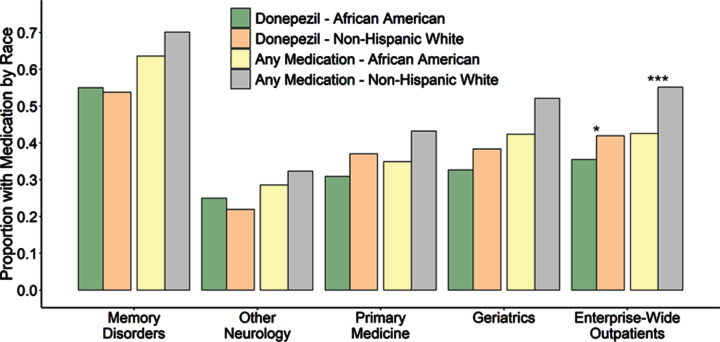
Fig.4.
Proportion of AD/MCI outpatients with prescriptions for donepezil individually (left) as well as combined with other FDA-approved AD treatments including galantamine, rivastigmine and memantine (right), subset by race and clinic. Outpatients seen by Memory Disorders specialists were significantly more likely to be using an antidementia medication compared to any other outpatient clinical setting individually or the entire medical system (p < 0.0001 for donepezil or all four FDA-approved medications for all settings, p-values not shown on plot). Within individual clinics, the African American proportion of AD/MCI patients using donepezil did not differ from the non-Hispanic white proportion of AD/MCI patients using donepezil or for any FDA-approved antidementia medication at any of the centers. However, due to the racial underrepresentation of African American AD/MCI outpatients in specialty settings and higher overall medication usage in Memory Disorders, both donepezil and overall medication use by African American AD/MCI patients was observed to be significantly lower than use by non-Hispanic whites when examined across the entire medical enterprise (p = 0.0035 and p < 0.0001 respectively). Although not indicated on this plot, memantine use for moderate to severe AD was lower for African Americans compared to non-Hispanic whites in Memory Disorders (p = 0.0008) and the Geriatrics clinics (p = 0.0070) as well as across the entire medical enterprise (p < 0.0001) when considered for all AD/MCI outpatients. However, within Memory Disorders, when specifically considering outpatients with Alabama Brief Cognitive Screener (ABCs) scores less than 14, indicative of moderate AD, there was no difference in the proportion of African Americans using memantine (37.0%) compared to non-Hispanic whites using the drug (45.1%, p = 0.53).