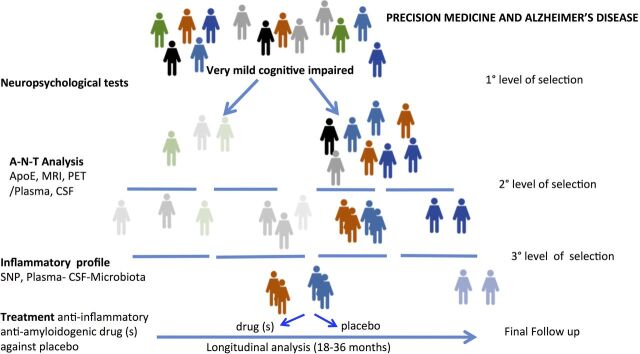
Figure 1.
Flow chart of hypothetical clinical trial protocol with three levels of selection of patients with mild cognitive impairment: (1) neuropsychological tests to distinguish amnestic and non-amnestic MCI (2) CSF analyses to identify subjects with high propensities to convert to AD and (3) inflammatory profiles assessed by the consideration of numerous parameters in plasma, CSF and microbiota, together with genetic background. In this model, the combination of antiamyloidogenic and anti-inflammatory drugs versus placebo is investigated. AD, Alzheimer’s disease; ApoE, apolipoprotein E, CSF, cerebrospinal fluid; MCI, mild cognitive impairment; PET, positron emission tomography; SNP, single nucleotide polymorphisms.