Figure 1.
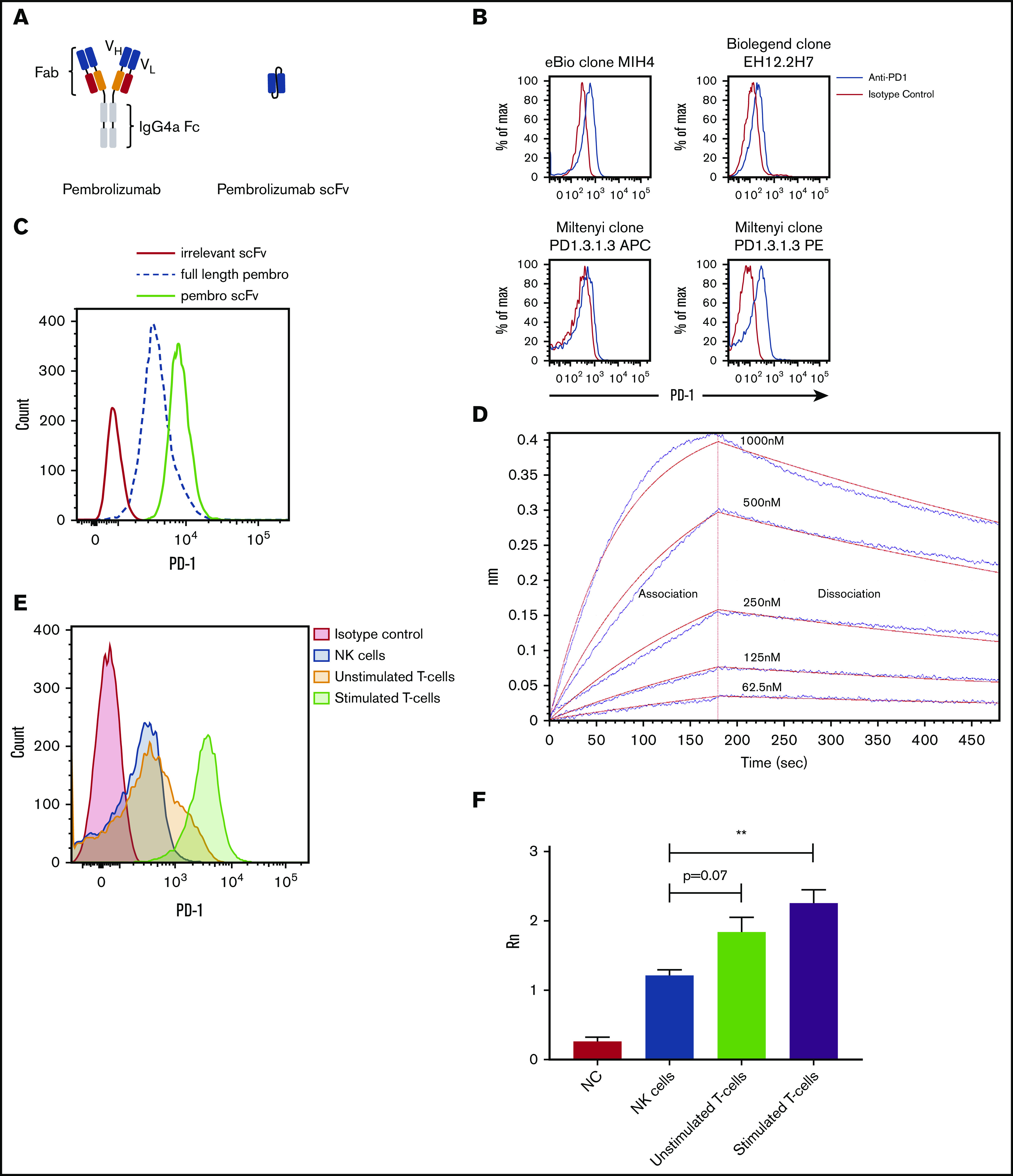
Schematic and binding of anti-PD-1 antibodies on primary NK cells. (A) Pembrolizumab is a complete mAb containing a human IgG4a Fc and 2 PD-1–specific Fab fragments compared with the scFv composed of the VH and VL chains of pembrolizumab connected by a short peptide linker. (B) PD-1 staining of healthy donor NK cells was assessed by using various commercially available antibodies. Shown is a representative example of 10 normal donors. (C) Flow cytometric analysis of PD-1 expression on peripheral blood NK cells from healthy donors is identified by the FITC-labeled pembrolizumab (pembro) or pembrolizumab scFv. (D) Confirmation of pembrolizumab scFv affinity for PD-1 is demonstrated by an Octet binding assay. (E) NK cells and T cells were isolated from healthy donor PBMCs by negative selection. T cells were left unstimulated or stimulated with anti-CD3 or anti-CD28 and IL-2 for 7 days and then stained for PD-1. (F) NK cells and resting and activated T cells were used for qPCR for PD-1 transcripts (n = 3). Error bars indicate the mean ± standard error of the mean (SEM). **P < .01. APC, fluorochrome allophycocyanin; NC, no template control.
